Magnetic particle chemiluminiscence detection kit for determining content of glycocholic acid in human body
A chemiluminescent reagent, the technology of glycocholic acid, which is applied in the direction of chemiluminescence/bioluminescence, and analysis by making materials undergo chemical reactions, can solve complex and cumbersome operations, gas chromatography and mass spectrometry are not suitable for clinical detection, ELISA method low degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] A method for preparing a magnetic particle chemiluminescence detection kit for measuring glycocholic acid content in human body, comprising the following steps:
[0056] Preparation of reagent R1: 1) Prepare buffer according to the content of reagent R1 buffer components, and adjust pH; 2) Prepare fluorescein isothiocyanate-labeled anti-glycocholic acid-BSA monoclonal antibody; 3) Fluorescent isothiocyanate Dilute the anti-glycocholic acid-BSA monoclonal antibody labeled with R1 buffer.
[0057] Preparation of reagent R2: 1) Prepare buffer according to the content of reagent R2 buffer components, adjust pH; 2) prepare alkaline phosphatase-labeled glycocholic acid antigen; 3) use alkaline phosphatase-labeled glycochlic acid antigen with R2 Buffer for dilution.
[0058] Preparation of magnetic separation reagents: 1) preparation of buffer solution according to the content of magnetic particle buffer components; 2) preparation of magnetic particle coated with anti-fluores...
Embodiment 1
[0076] Example 1 A magnetic particle chemiluminescence detection kit for determining the content of glycocholic acid in human body includes R1 reagent, R2 reagent, magnetic separation reagent, calibrator solution series and substrate solution.
[0077] In this example, the R1 reagents include: 1) R1 antibody: fluorescein isothiocyanate (FITC)-labeled anti-glycocholic acid-BSA monoclonal antibody, the concentration is 0.3 μg / ml; 2) buffer: Tris, the concentration 12.04g / L; sodium azide, the concentration is 1.987g / L; sodium chloride, the concentration is 5.79g / L, bovine serum albumin, the concentration is 9.86g / L; 8 aniline-1-naphthalenesulfonate ammonium salt , the concentration is 0.986g / L; the rest is deionized water. The buffer pH of the R1 reagent is preferably 7.0.
[0078] In this embodiment, the R2 reagents include: 1) R2 antibody: alkaline phosphatase-labeled glycocholic acid antigen at a concentration of 0.5 μg / ml; 2) buffer: commercially available AP Conjugate Stabi...
Embodiment 2
[0082] Example 2 Preparation and determination method of magnetic particle chemiluminescence detection kit for measuring glycocholic acid content in human body
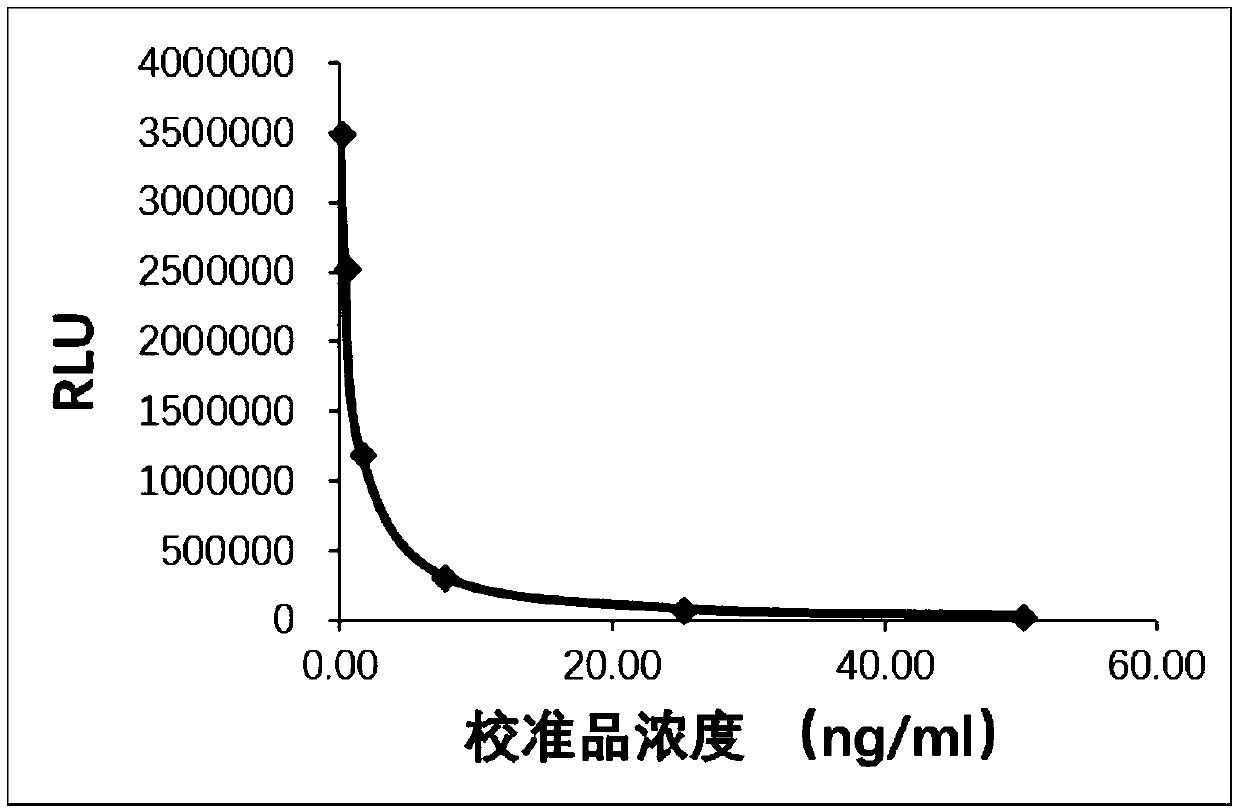
[0083] (1) First, add the 50 μl calibrator series of Example 1 (concentrations are 0, 0.33, 1.5, 7.5, 25, 50 ng / ml) and 50 μl reagent R1 and 50 μl reagent R2 of Example 1 respectively into the reaction tube medium, mix and incubate at 37°C for 15 minutes;
[0084] (2) Combine the above reagent series with 25 μl of the magnetic separation reagent of Example 1, and then continue to incubate at 37° C. for 5 min;
[0085] (3) Wash 3 times with cleaning solution to remove unbound antibodies and impurities;
[0086] (4) Add 150 μl of the luminescence substrate solution in Example 1, and use the Leadman self-developed chemiluminescence detector to measure the relative luminescence intensity (RLU) after ALP catalyzes the substrate luminescence. The results are shown in the following table:
[0087] Table 1
[0088] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com