Tumor-associated antigen 153 magnetic microparticle chemiluminescence immune assay determination kit and method for making same
A tumor-associated antigen and chemiluminescence technology, which is applied in the field of immunoanalysis medicine, can solve the problems of low sensitivity, short storage time of reagents, and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of Tumor-associated Antigen 153 Magnetic Particle Chemiluminescence Immunoassay Assay Kit of the present invention
[0042] 1. Preparation of Tumor-Associated Antigen 153 Calibrator
[0043] Dilute the pure product of tumor-associated antigen 153 with horse serum into calibrator, the concentrations are 0U / mL, 5U / mL, 15U / mL, 50U / mL, 150U / mL, 500U / mL, 6 bottles in total.
[0044] 2. Preparation of a mixture of tumor-associated antigen-153 monoclonal antibody-coated magnetic particles and biotin-labeled tumor-associated antigen-153 monoclonal antibody
[0045] 1. Preparation of magnetic particles coated with tumor-associated antigen 153 monoclonal antibody
[0046] Activate the magnetic particles with a particle size of 1-2 μm with glutaraldehyde, stir at room temperature, mix for 3 hours, apply a magnetic field, let stand for 20-25 minutes, pour out the supernatant, and use 0.01mol / L with a pH value of 7.4 Wash three times with phosphate buffer, an...
Embodiment 2
[0069] Embodiment 2 The usage method of the kit of the present invention
[0070] 1. Sample pretreatment
[0071] Take human fasting morning serum samples, centrifuge at 3000rpm for 5min, and take 50μL of supernatant for analysis.
[0072] 2. Detection method
[0073] Before using this kit for experiments, take out the mixture of antibody-coated magnetic particles and biotin-labeled antibodies, calibrator / sample to be tested, and enzyme-labeled avidin and place them at room temperature for 15-30 minutes to allow them to balance to Room temperature; after that, adjust the incubator or water bath to 37°C; then, prepare a suitable micro-sampler and corresponding tips and check whether the chemiluminescence instrument is working normally.
[0074] The specific operation steps of using this kit to carry out the experiment according to the method of embodiment 1 are as follows:
[0075] After numbering the round-bottom polystyrene test tubes, add 50 μL of serum samples or serial ...
Embodiment 3
[0076] The methodological examination of the kit of the present invention of embodiment 3
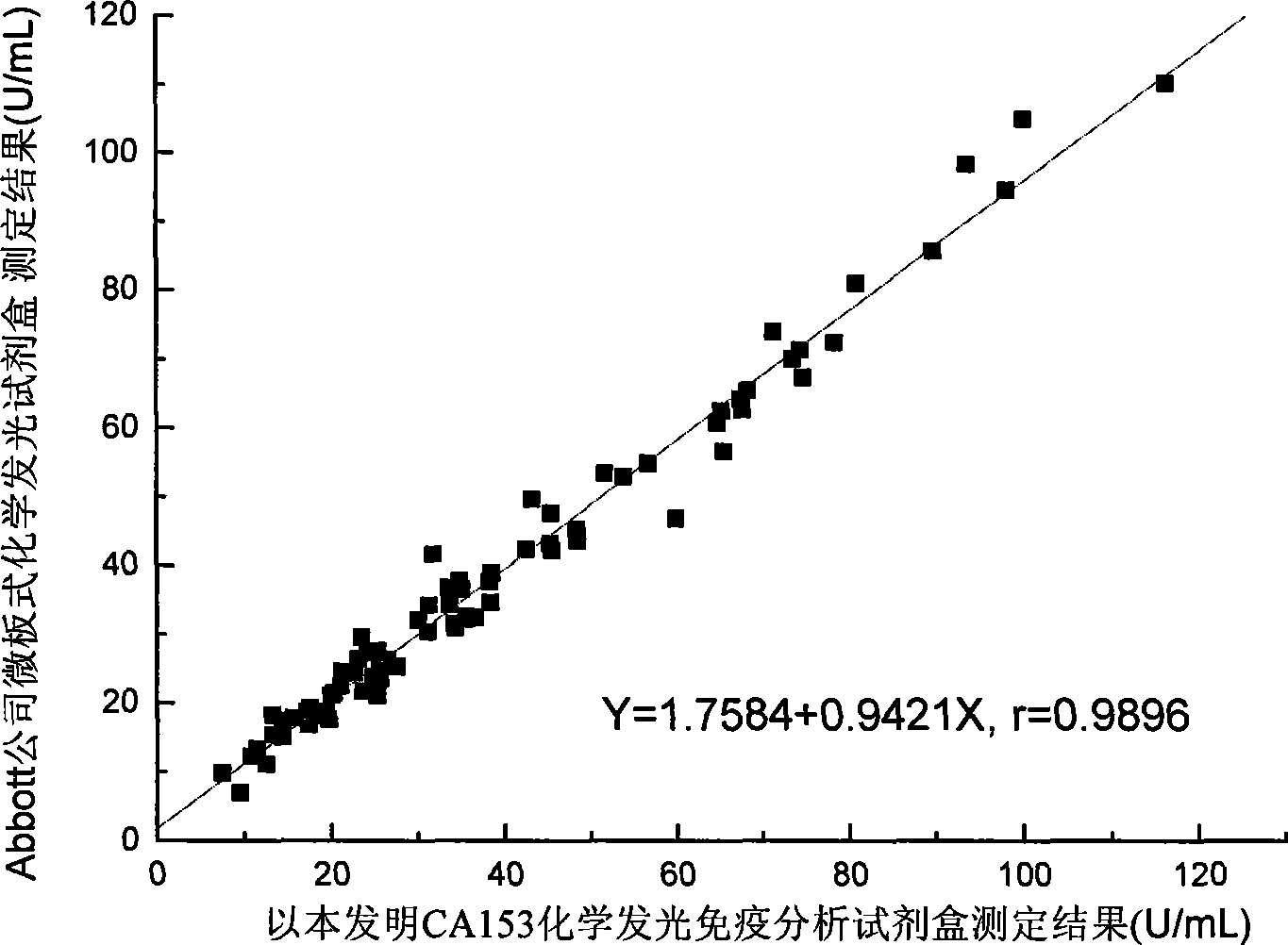
[0077] The test kit prepared in Example 1 is tested according to the conventional manufacturing and testing procedures in the art, and the results are as follows:
[0078] 1. Determination of kit precision
[0079] (1) Calibrator precision experiment
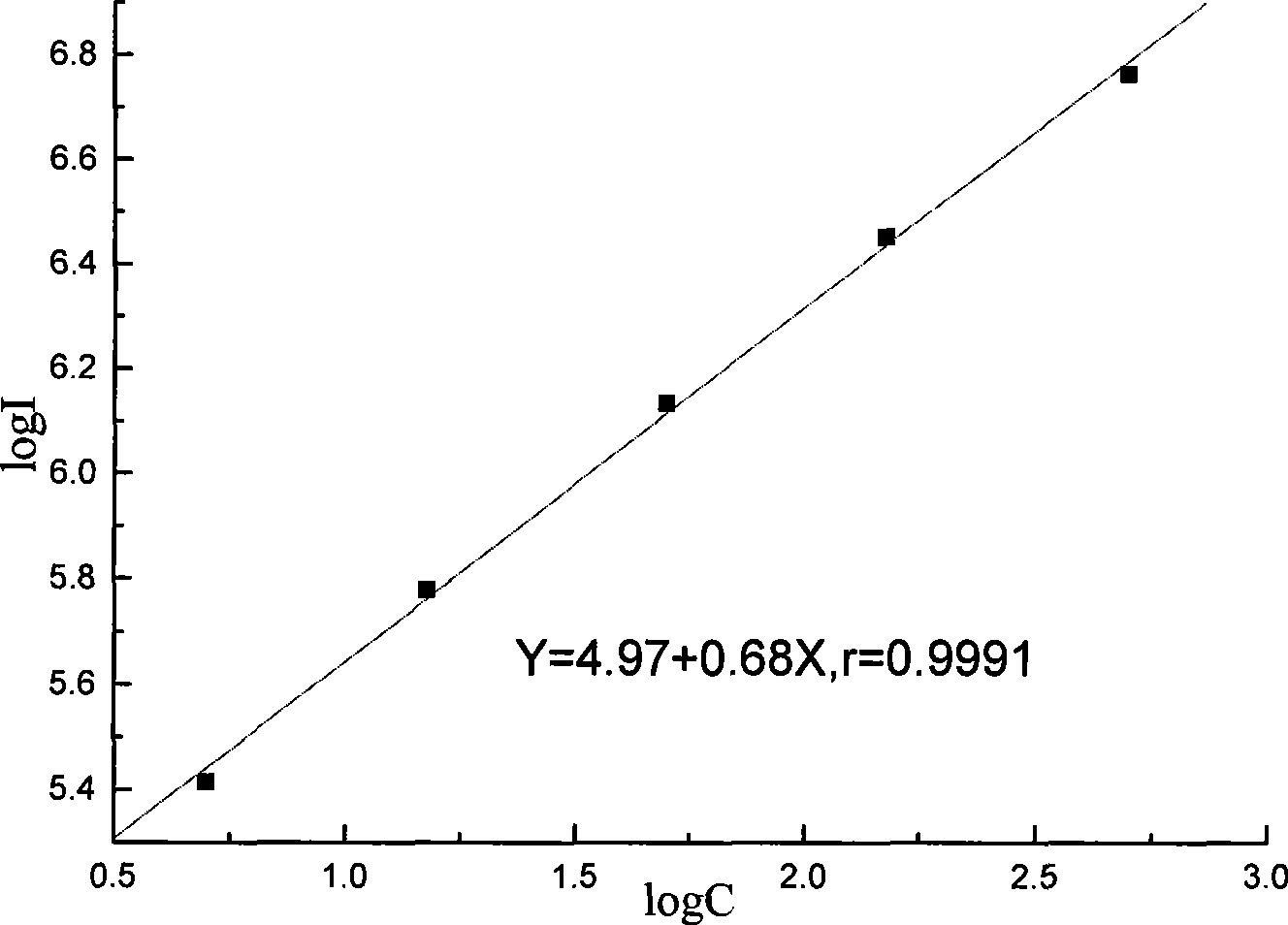
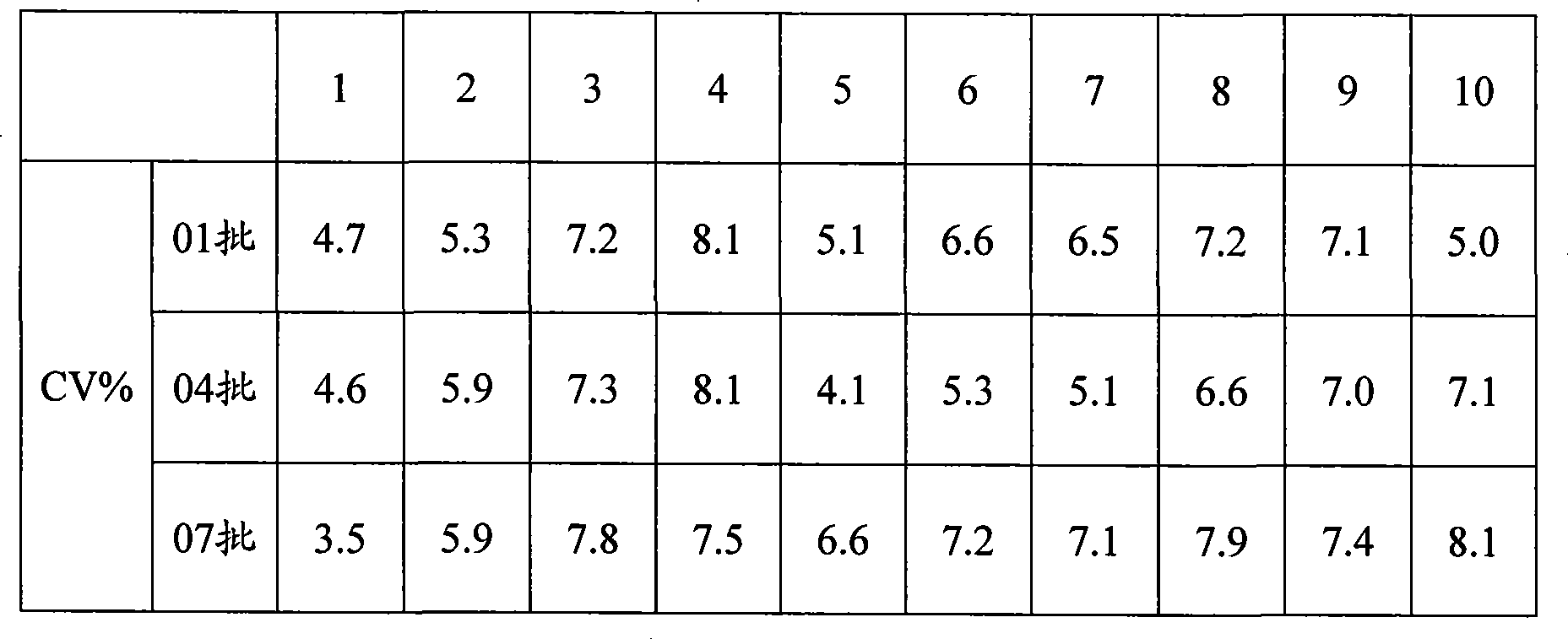
[0080] Three batches of the kits prepared in Example 1 were taken for the precision test, and 10 kits were taken from each batch. Measure the 50 U / mL CA153 calibrator 5 times with the kit extracted in Example 1. Calculate the coefficient of variation for the measured concentrations. The measurement results of the three batches of kits in Example 1 are shown in Table 1, and the results show that the coefficient of variation is between 3.5% and 8.1%.
[0081] Table 1 CA153 Calibration Repeatability Experiment
[0082]
[0083] (2) Sample repeatability experiment
[0084] Take two normal human serum, add CA153 calibrator to 50U / mL a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com