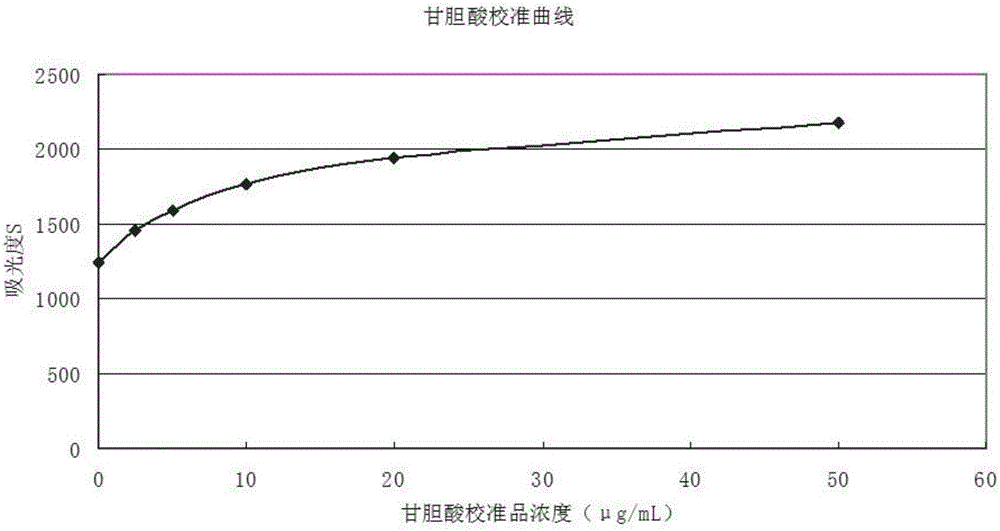
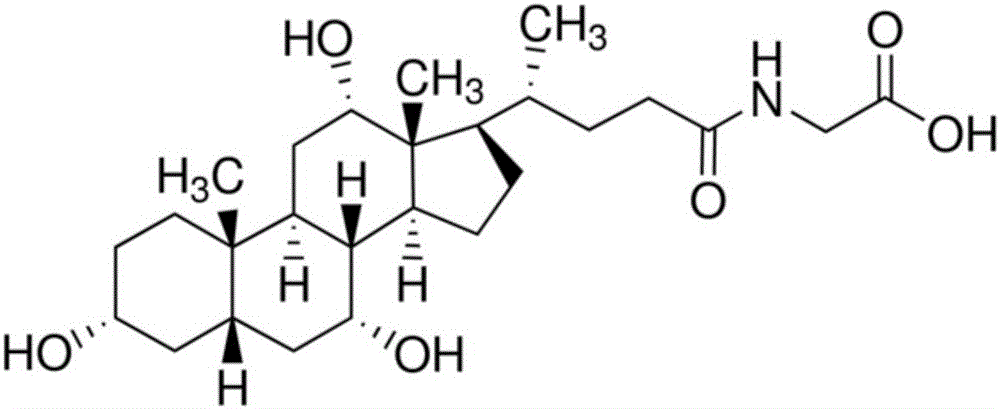
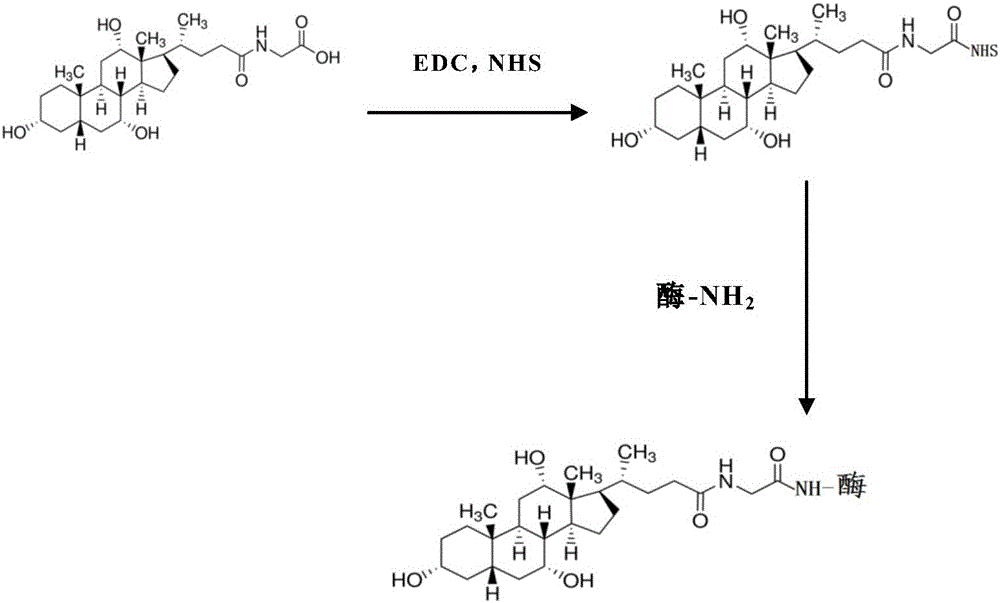
Preparation method for homogeneous enzyme immunodiagnosis reagent used for glycocholic acid
A technology for a diagnostic reagent and a glycocholidase conjugate, which is applied in the field of biomedicine, can solve the problems of unfavorable clinical tests, poor accuracy of test results and reagent stability, complicated operation, etc., and achieves a simple and easy-to-control preparation process and high-efficiency reagents. Accurate and sensitive, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A preparation method of glycocholic acid homogeneous enzyme immunodiagnostic reagent:
[0043] (1) prepare glycocholic acid antibody solution,
[0044] Add glycocholic acid antibody to Tris-HCl buffer solution with pH 7.4, add 6-phosphate glucose, NADP, BSA, sodium azide, and mix well to obtain glycocholic acid antibody solution. The concentration of the glycocholic acid antibody in the solution is 0.05 mg / ml, and the mass percent content of sodium azide is 0.1%;
[0045](2) preparing a glycocholidase conjugate solution,
[0046] Add glycocholic acid to MES buffer, add carboxyl activator EDC-NHS for carboxyl activation, carboxyl activation temperature is 0°C, time is 5min, pH is 5.5, then add 6 -Phosphoglucose dehydrogenase carries out condensation reaction to obtain glycocholidase conjugate crude product, the mass ratio of 6-phosphoglucose dehydrogenase and glycocholic acid is 1:1000, the glycocholidase conjugate crude product is at pH The glycocholate enzyme conjug...
Embodiment 2
[0050] A preparation method of glycocholic acid homogeneous enzyme immunodiagnostic reagent:
[0051] (1) prepare glycocholic acid antibody solution,
[0052] Add the glycocholic acid antibody to the Tris-HCl buffer solution with a pH of 8.0, add 6-phosphate glucose, NADP, sucrose, and sodium azide, and mix evenly to obtain the glycocholic acid antibody solution. Among them, glycocholic acid The concentration of the antibody is 0.2mg / ml, and the mass percentage content of sodium azide is 0.2%;
[0053] (2) preparing a glycocholidase conjugate solution,
[0054] Add glycocholic acid to MES buffer, add carboxyl activator EDC-NHS for carboxyl activation, carboxyl activation temperature is 10°C, time is 15min, pH is 6.0, then add 6 -Phosphoglucose dehydrogenase carries out condensation reaction to obtain glycocholidase conjugate crude product, wherein, the mass ratio of 6-phosphoglucose dehydrogenase and glycocholic acid is 1:10, and the glycocholidase conjugate crude product is...
Embodiment 3
[0058] A preparation method of glycocholic acid homogeneous enzyme immunodiagnostic reagent:
[0059] (1) prepare glycocholic acid antibody solution,
[0060] Add the glycocholic acid antibody to the Tris-HCl buffer solution with a pH of 8.5, add 6-phosphate glucose, NADP, mannose, and sodium azide, and mix evenly to obtain the glycocholic acid antibody solution. The concentration of the acid antibody is 5mg / ml, and the mass percent content of sodium azide is 0.3%;
[0061] (2) preparing a glycocholidase conjugate solution,
[0062] Add glycocholic acid to MES buffer, add carboxyl activator EDC-NHS for carboxyl activation, carboxyl activation temperature is 20°C, time is 25min, pH is 6.5, then add 6 -Phosphoglucose dehydrogenase carries out condensation reaction to obtain glycocholidase conjugate crude product, wherein, the mass ratio of 6-phosphoglucose dehydrogenase and glycocholic acid is 1:1, and the glycocholate enzyme conjugate crude product is in The glycocholidase c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com