High-purity tauro ursodesoxy cholic acid and preparation method thereof
A technology of tauroursodeoxycholic acid and tauroursodeoxycholic acid, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, steroids, etc., can solve the hidden safety hazards of tauroursodeoxycholic acid , can not effectively remove bezoar chenodeoxycholic acid and other problems, to achieve the effect of suitable for industrial production, low cost and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In this experiment, the liver toxicity of tauroursodeoxycholic acid samples containing different taurochenodeoxycholic acid impurities was studied.
[0058] 1. Materials and Methods
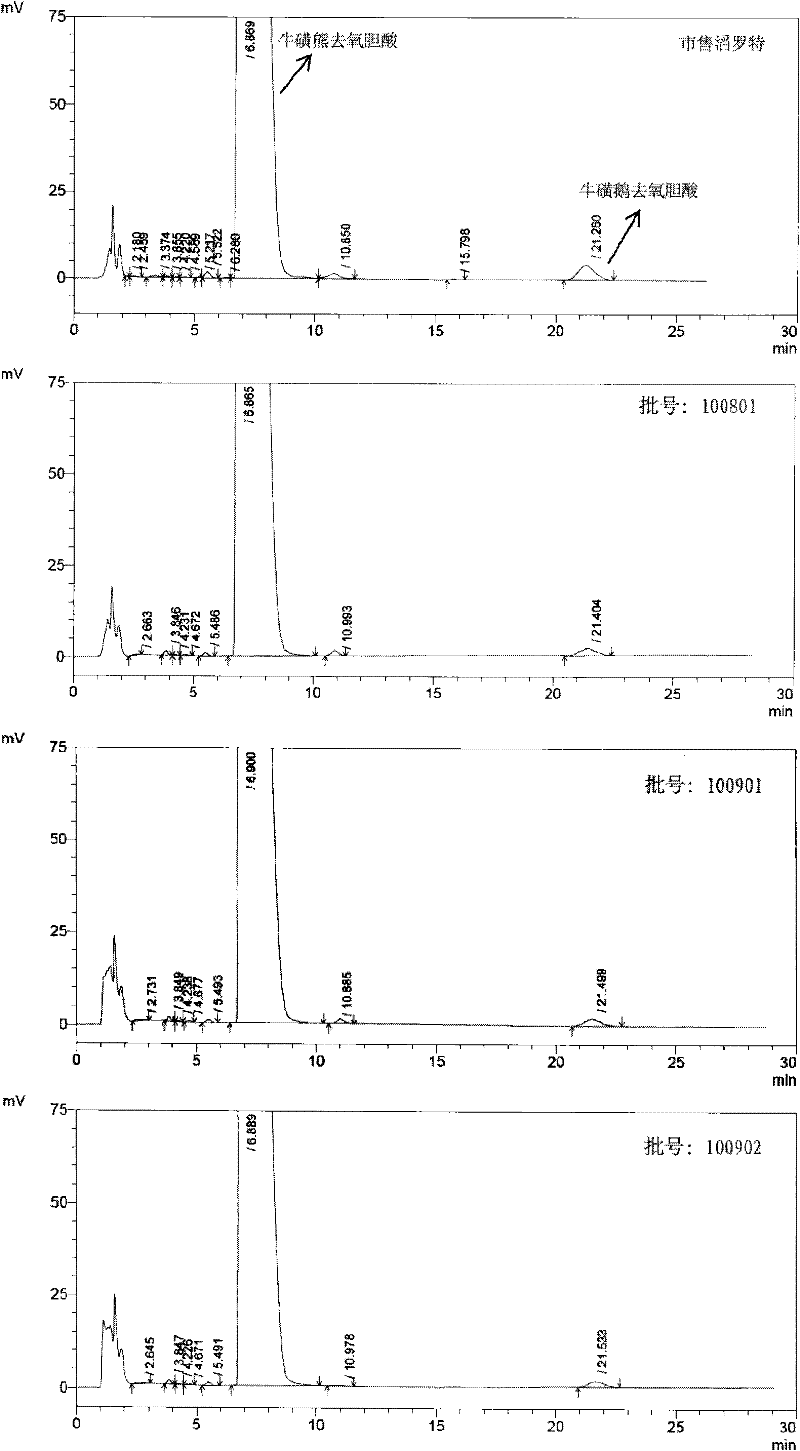
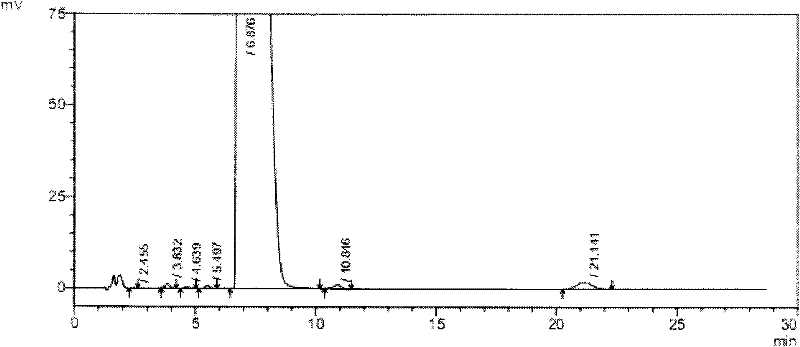
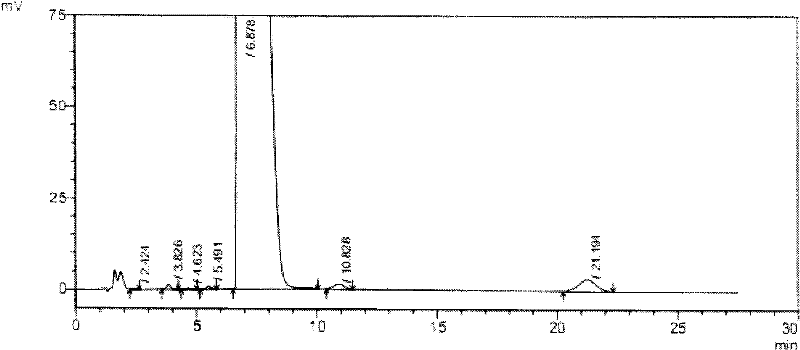
[0059] 1.1 Test drug: tauroursodeoxycholic acid containing 2% taurourchenodeoxycholic acid impurity (batch number: 100810), white powder, self-made; Sulfurursodeoxycholic acid (batch number: 100811), white powder, self-made; tauroursodeoxycholic acid (batch number: 100812), white powder, containing 0.7% taurodeoxycholic acid impurity; Tauroursodeoxycholic acid (batch number: 100813) with 0.5% impurity content of sulchenodeoxycholic acid, white powder, self-made; the above samples are all insoluble in water.
[0060] 1.2 Detection reagents: Liver function-related detection kits were purchased from Sichuan Mike Biotechnology Co., Ltd., mainly including: alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
[0061] 1.3 Detection instrument: full-wavelength fluorescence and s...
Embodiment 2
[0077] The preparation of embodiment two high-purity tauroursodeoxycholic acid
[0078] a) The preparation of the crude product of tauroursodeoxycholic acid is carried out according to the following steps:
[0079] In the reaction kettle, add 4.0KG ursodeoxycholic acid, add 19KG acetone, add 1650ml triethylamine under stirring, turn on the jacket refrigeration, and lower the temperature of the mixture to -10°C. Add 1120ml of ethyl chloroformate, control the rate of addition of ethyl chloroformate, so that the temperature of the reaction solution is maintained between -5-0°C, the addition is completed in 30 minutes, continue to maintain the temperature and stir for 40 minutes to obtain ursodeoxy For the mixed anhydride of cholic acid and ethyl chloroformate, the above mixed anhydride reaction solution is filtered through a filter with nitrogen pressure to be equipped with 4.2KG purified water, 1.4KG taurine, and 0.4KG sodium hydroxide in a 50-liter reactor. The reaction was st...
Embodiment 3
[0084] The preparation method of embodiment three high-purity tauroursodeoxycholic acid
[0085] a) The preparation of the crude product of tauroursodeoxycholic acid is carried out according to the following steps:
[0086] In the reaction kettle, add 40.0KG of ursodeoxycholic acid, add 190KG of acetone, add 16.5L of triethylamine under stirring, turn on the jacket refrigeration, and lower the temperature of the mixture to -5°C. Add 11.2 L of ethyl chloroformate, control the rate of addition of ethyl chloroformate, so that the temperature of the reaction solution is maintained between 3-5 °C, the addition is completed in 30 minutes, and the temperature is continued to be stirred for 30 minutes to obtain ursodeoxy For the mixed acid anhydride of cholic acid and ethyl chloroformate, the above mixed anhydride reaction solution is filtered through a filter with nitrogen pressure into a 500-liter reactor equipped with 42KG purified water, 14KG taurine, and 4KG sodium hydroxide, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com