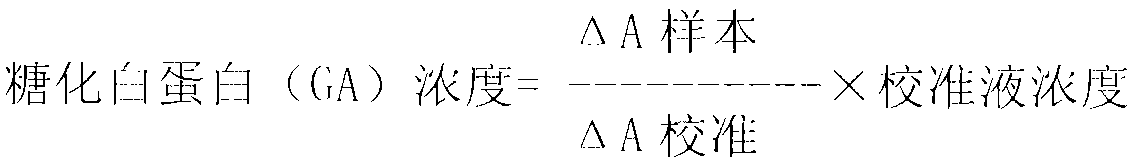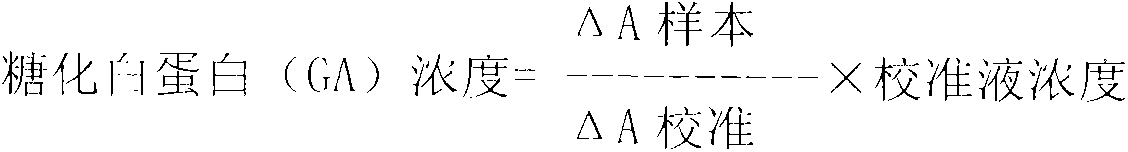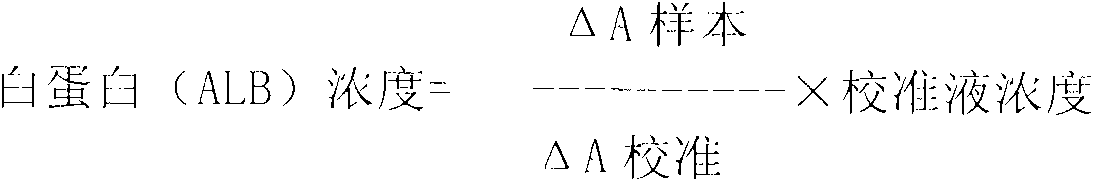Patents
Literature
274 results about "Choleic Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
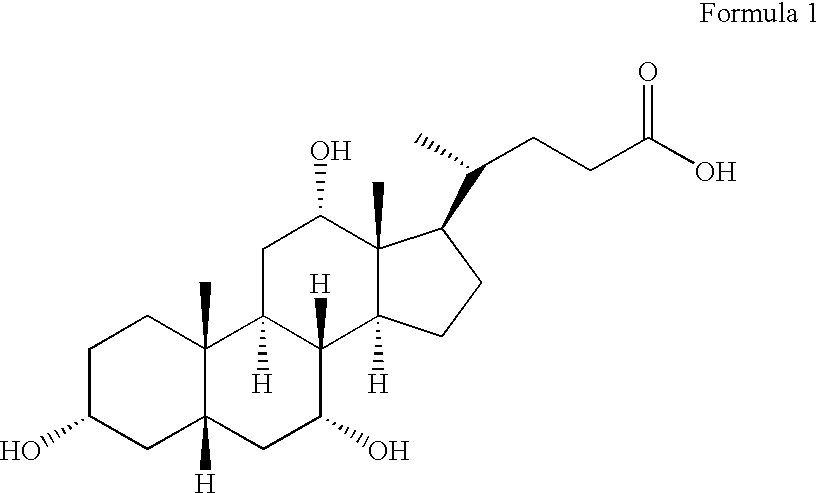
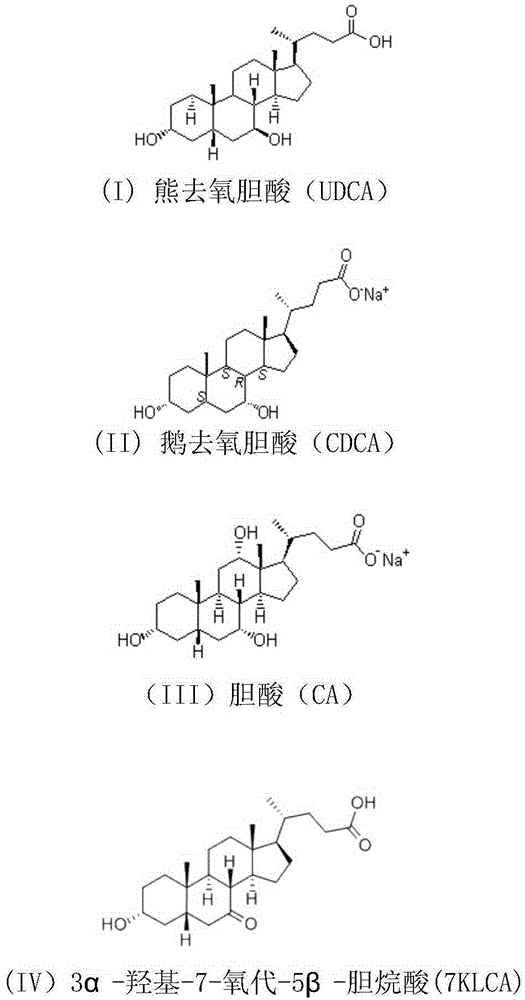
Cholic acid, also known as 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid is a primary bile acid that is insoluble in water (soluble in alcohol and acetic acid), it is a white crystalline substance. Salts of cholic acid are called cholates.
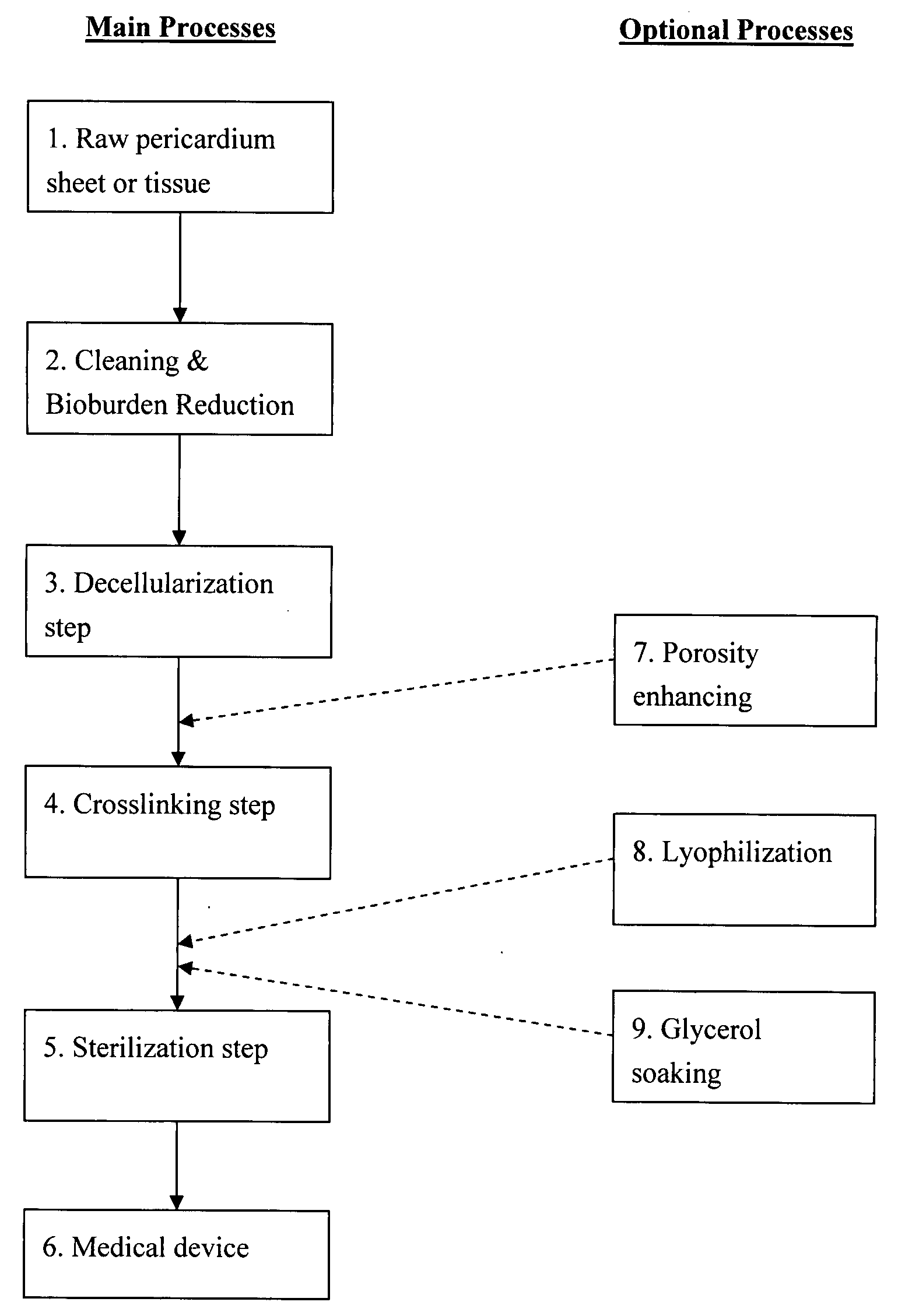
Decellularized pericardial tissue
InactiveUS20080195229A1Low antigenicityLow immunogenicityTissue regenerationProsthesisChemical treatmentPericardium
The invention discloses a decellularized pericardial tissue via chemical treatment with cholic acid or bile salts as a medical device and process of manufacture.
Owner:QUIJANO RODOLFO C +1
Valved stent for chronic venous insufficiency
InactiveUS20090254175A1Minimize turbulenceReduce molecular weightVenous valvesBlood vesselsChemical treatmentCoupling
The invention discloses a valved stent and process of manufacture for treating chronic venous insufficiency having the geometry of the supporting frame and its coupling to the membrane of a specific geometry that provides the valvular mechanism for optimal function. The membrane may comprise a decellularized pericardial tissue via chemical treatment with cholic acid or bile salts and crosslinked.
Owner:QUIJANO RODOLFO C +1
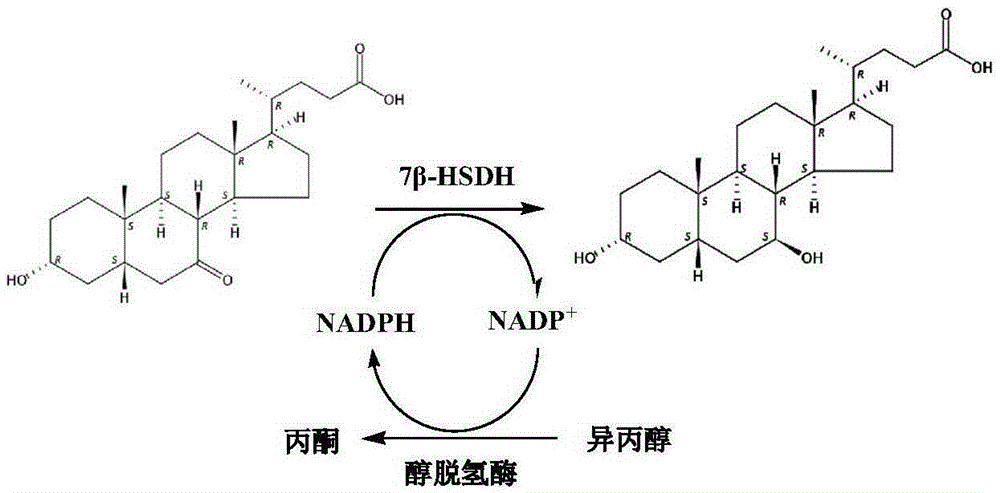
Mutant of 7 beta-hydroxyl steroid dehydrogenase, application of mutant and synthesis method
ActiveCN105274070ASuitable for industrial productionEasy to controlOxidoreductasesFermentationChemical synthesisCholic acid
The invention provides a mutant of 7 beta-hydroxyl steroid dehydrogenase, application of the mutant and a synthesis method. The mutant of the 7 beta-hydroxyl steroid dehydrogenase is characterized in that amino acid sequences of the mutant are Seq ID NO:4, and coded nucleotide sequences are Seq ID NO:3; or amino acid sequences of the mutant are Seq ID NO:6, and coded nucleotide sequences are Seq ID NO:5. The mutant, the application and the synthesis method have the advantages that cholic acid compounds, particularly ursodeoxycholic acid, can be catalytically synthesized by the efficient 7 beta-hydroxyl steroid dehydrogenase, mutant enzymes of the 7 beta-hydroxyl steroid dehydrogenase and coenzyme regeneration systems, accordingly, the substrate concentration can reach 100 g / L, the conversion rate is 99.2-99.5%, and the weight yield can reach 94-96%; and the enzymes can be inexpensively and easily obtained by the aid of a fermentation process, accordingly, the production cost and the product quality are superior to the production cost and the product quality of chemical synthesis methods, and the mutant and the synthesis method are applicable to industrial production.
Owner:苏州天绿生物制药有限公司
High efficiency technique for extracting bilirubin and bile acid by using animal bile as raw material
The present invention relates to process of extracting cholic acid, deoxycholic acid and bilirubin from bile of pig, ox and sheep. Bile of pig, ox and sheep consists of water in about 97 %, bile acid in about 2.5 % and bilirubin in about 0.4 %; and contains also phospholipid, cholesterol, Na, K, Ca, phosphate, carbonate, small amount of protein, and other components. Fresh bile is treated through cooling, filtering to defat, basic hydrolysis, acidification and organic solvent extracting to obtain bilirubin; the rest solution is further treated through deep saponification, acidification and organic solvent precipitation to obtain the mixture of cholic acid and deoxycholic acid; and the mixture is re-crystallization separated to obtain high purity cholic acid and deoxycholic acid.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Separation purification preparation method of chenodeoxycholic acid in pig's bile
InactiveCN1869044ASimple and fast operationLow costUnknown materialsSteroidsCholic acidChenodeoxycholic acid
A process for separating the chenodeoxycholic acid from pig's gall and purifying it includes such steps as preparing general cholic acid from the mother liquid generated by extracting the cholerythrin from pig's gall, saponifying, regulating pH value to obtain crude chenodeoxycholic acid, decoloring, defatting, preparing the deposit of barium chenodeoxycholate, reacting on potassium carbonate to remove Ba, regulating pH value, and purifying by silicon gel column.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
High-purity tauro ursodesoxy cholic acid and preparation method thereof
ActiveCN102477059AHigh purityImprove securityOrganic active ingredientsDigestive systemEthyl chloroformateCholic acid
The invention relates to high-purity tauro ursodesoxy cholic acid and a preparation method thereof. The content of taurochenodeoxycholic acid in the tauro ursodesoxy cholic acid is less than 0.7%. The tauro ursodesoxy cholic acid is safe and effective and does not have toxic and side effects in clinical application. The invention further provides a mixed anhydride reaction of ursodesoxycholic acid and ethyl chloroformate by taking acetone as a solvent. By means of control of a reaction condition and a reaction solvent, the tauro ursodesoxy cholic acid has the advantages of simple process, low cost, environmental friendlessness and industrial production; furthermore, the high-purity tauro ursodesoxy cholic acid can be obtained.
Owner:CHENGDU GUOHONG PHARMA
Compositions and methods for therapeutic use
A method and pharmaceutical composition for the enhancement of transfer of a therapeutic agent to a cell wherein the therapeutic agent is formulated in a buffer comprising a compound of Formula I:wherein:n is an integer from 2-8; X1 is a cholic acid group or deoxycholic acid group; and X2 and X3 are each independently selected from the group consisting of a cholic acid group, a deoxycholic acid group, and a saccharide group, wherein the saccharide group is selected from the group consisting of pentose monosaccharide groups, hexose monosaccharide groups, pentose-pentose disaccharide groups, hexose-hexose disaccharide groups, pentose-hexose disaccharide groups, and hexose-pentose disaccharide groups; and wherein at least one of X2 and X3 is a saccharide group.
Owner:MERCK SHARP & DOHME CORP
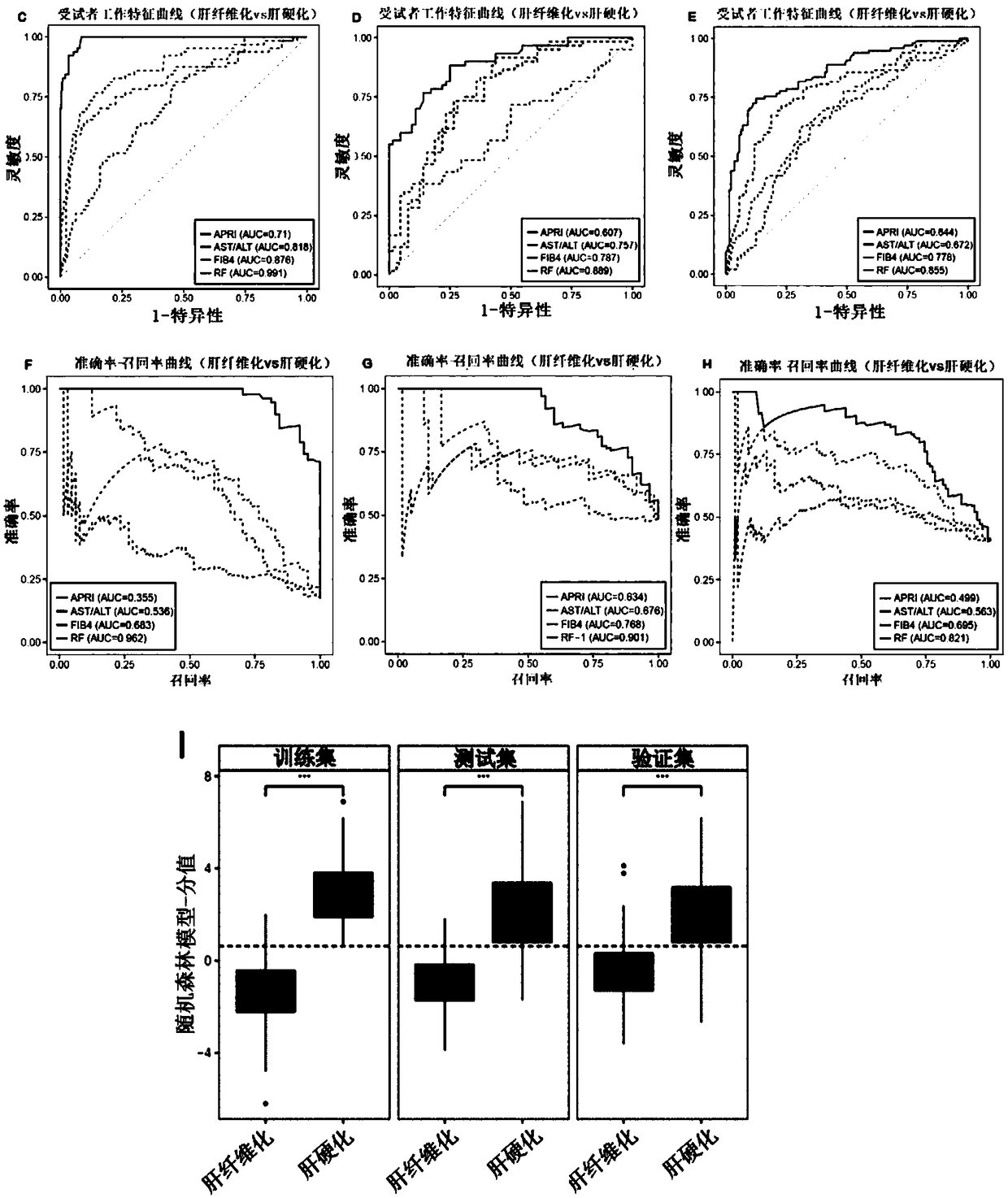
Biomarkers and kits for diagnosis of liver fibrosis and cirrhosis and use method
The invention provides a group of biomarkers which can be used for detecting liver fibrosis and cirrhosis. The biomarkers refer to metabolite components existing in a biological sample of a subject body and comprise a plurality of metabolites, wherein the metabolites are selected from cholic acid, amino acid and fatty acid. Metabolite combination comprises at least one amino acid, fatty acid and cholic acid, the metabolite combination is differentially expressed in at least one target plasma or serum and in a control plasma or serum. The biomarkers can optionally be combined with clinical indicators for diagnosis of liver fibrosis in the subject body. The biomarker composition has the characteristics of high sensitivity and specificity for pathological staging diagnosis of liver fibrosis patients, and can be clinically applied as a non-invasive means to improve clinical diagnosis and reduce puncture pain of the patients. The invention further provides a use method of the biomarkers andkits containing the biomarkers.
Owner:HUMAN METABOLOMICS INST INC
Method for preparing resistant starch of corn
InactiveCN101427741AGood physical and chemical propertiesDelicate tasteFood preparationFatty acidFermentation
The invention relates to a preparation method of corn resistant starch, which uses common corn starch as raw material to produce corn resistant starch with multiple physiological functions and characteristics by combined treatment of microbial fermentation and hot pressing and cooling. The resistant starch has physiologic functions of effectively controlling body weight, preventing constipation, appendicitis, hemorrhoids and colon cancer, controlling diabetes, increasing lipid discharge, improving lipid composition, promoting absorption of zinc, calcium and magnesium ions, increasing removal of cholesterol and cholic acid, and reducing cholesterol biosynthesis, lipid absorption and fatty acid biosynthesis. The resistant starch used in food processing industry has better physicochemical properties than those of dietary fiber, low water retention capacity, good mouthfeel, no influence on food, and improve food texture. The invention solves the problems in production of resistant starch, including low yield and lack of high amylase starch in China.
Owner:JILIN AGRICULTURAL UNIV
Compositions and methods for therapeutic use
A method and pharmaceutical composition for the enhancement of transfer of a therapeutic agent to a cell wherein the therapeutic agent is formulated in a buffer comprising a compound of Formula I: wherein: n is an integer from 2-8; X1 is a cholic acid group or deoxycholic acid group; and X2 and X3 are each independently selected from the group consisting of a cholic acid group, a deoxycholic acid group, and a saccharide group, wherein the saccharide group is selected from the group consisting of pentose monosaccharide groups, hexose monosaccharide groups, pentose-pentose disaccharide groups, hexose-hexose disaccharide groups, pentose-hexose disaccharide groups, and hexose-pentose disaccharide groups; and wherein at least one of X2 and X3 is a saccharide group.
Owner:MERCK SHARP & DOHME CORP
Prepn process of Qingkailing injection and injection powder and its quality control method
The present invention provides a improved preparation process of Qingkailing injection and powder for injection. The improved preparation process includes high speed centrifugal treatment in extracting cape jasmine, isatis root and honeysuckle, merging extractive liquid, ultrafiltering and other technological steps. It can produce Qingkailing injection product and powder product for injection with ever higher stability and ever longer effective period. The present invention also discloses the quality control method of baicalin, cholic acid, hyodeoxycholic acid, jasminoidin and chlorogenic acid in Qingkailing injection and powder for injectino as well as identification method of Qingkailing injection and power for injection.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Quality detection method of Zhenlong brain-refreshment preparation
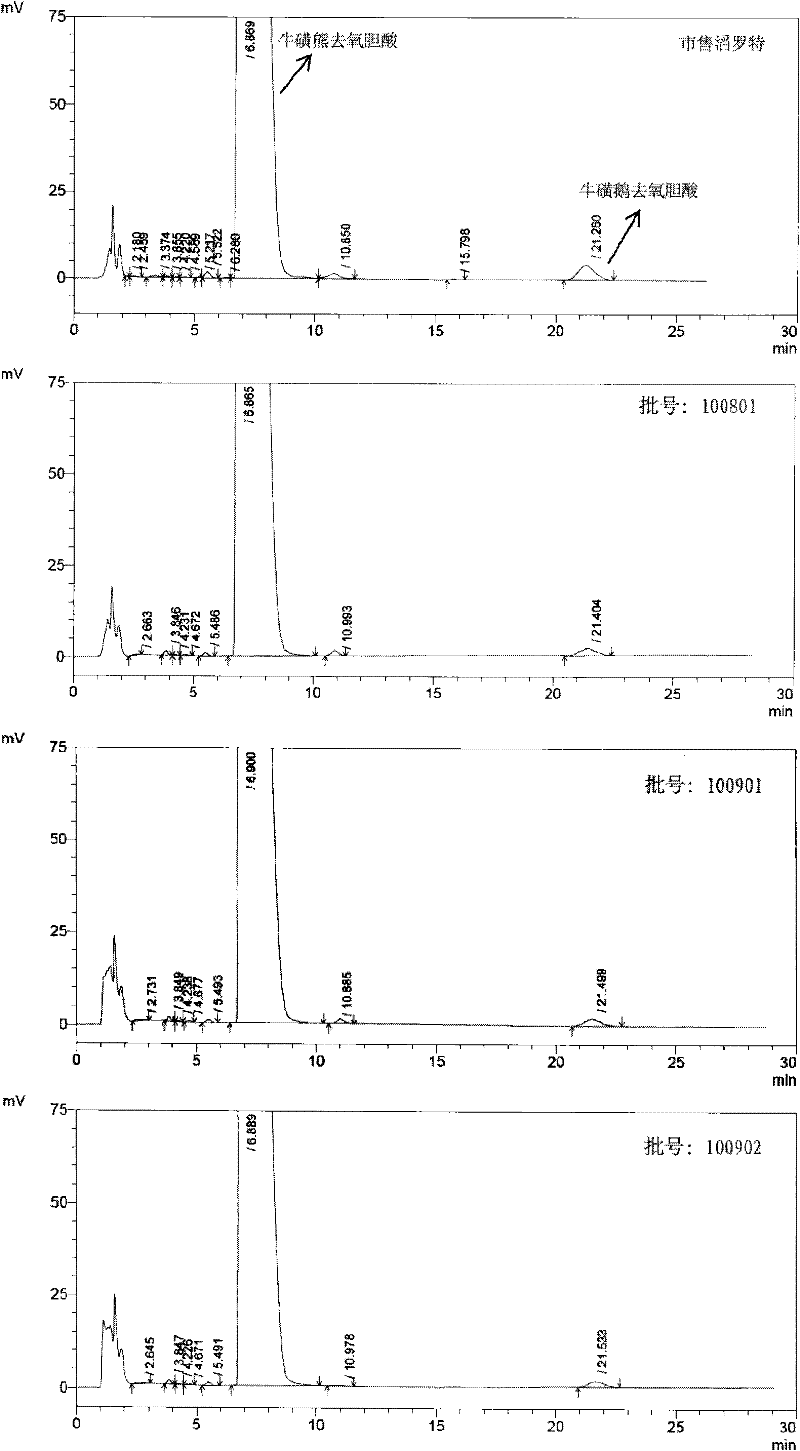
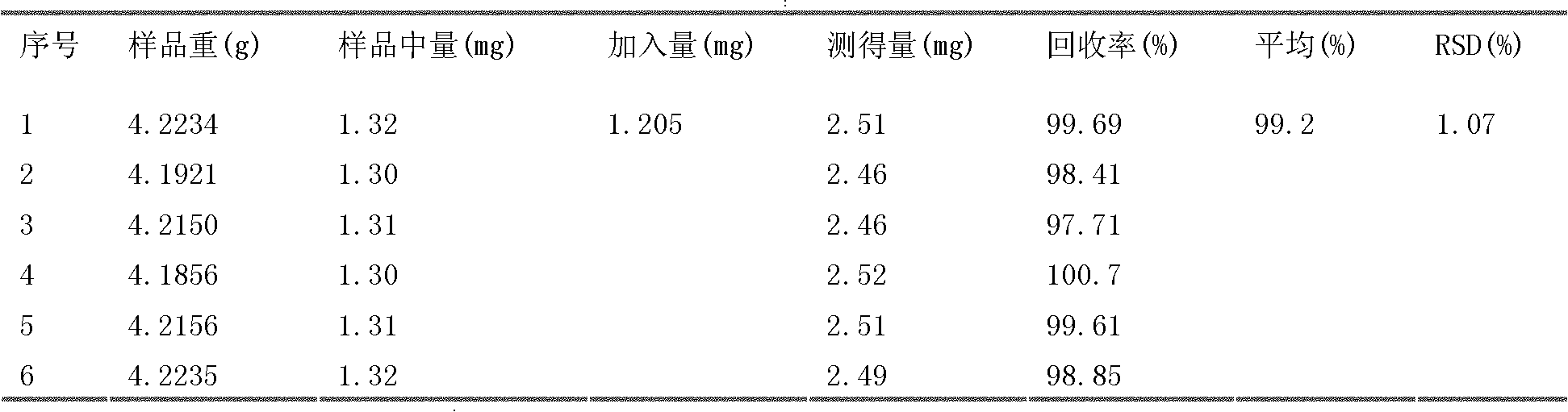
The invention discloses a quality detection method of a Zhenlong brain-refreshment preparation. The method provided by the invention comprises a discrimination method of piper longum, banksia rose, Chinese cassia tree, gallic acid, cardamom, clove, pearl, cholic acid, stigma croci and cinnamaldehyde as well as a discrimination method of piperine in piper longum, cholic acid in cow-bezoar, cinnamaldehyde in Chinese cassia tree, and crocin-I and crocin-II in stigma crociand. Through a test research, by the discrimination methods in the quality detection method of the test article chromatograph,spots with the same color are shown on the corresponding position of control article chromatograph without the interference of negative control. The method is convenient and feasible with strong character. The content detection method provided by the invention is used to detect the contents of piperine, cholic acid, crocin-I and crocin-II in the Zhenlong brain-refreshment preparation. The result shows that the method has good linear relation, high accuracy of a recovery test and good repeatability and stability, and can be used to accurately and rigorously control the quality of the Zhenlong brain-refreshment preparation.
Owner:JINHE TIBETAN MEDICINE
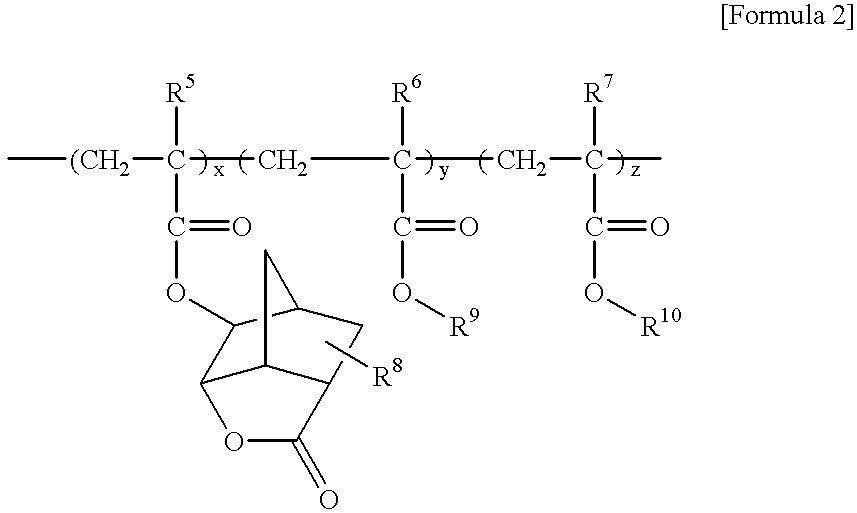
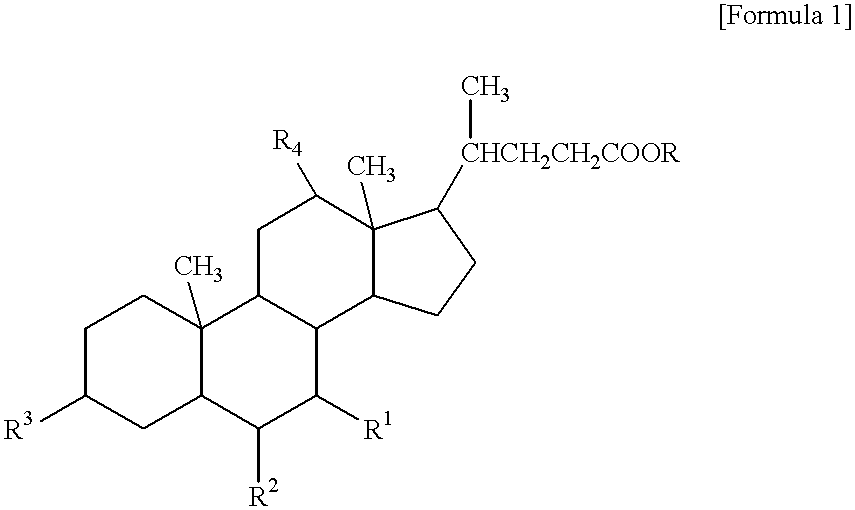
Positive chemically amplified resist and method for forming its pattern
InactiveUS20020042018A1Preventing patternIncrease stickinessPhotosensitive materialsSemiconductor/solid-state device manufacturingResistCholeic Acid
A form-improvement agent, for resist pattern, including a steroid compound is included in a positive chemically amplified resist. The form-improvement agent is in a range from 0.5 to 8 parts by weight per 100 parts by weight of a resin for resist included in the positive chemically amplified resist. The steroid compound is, for example, a cholic acid ester.
Owner:NEC CORP
Quality standard of throat smoothing dropping pills
InactiveCN101152241AImprove quality levelHydroxy compound active ingredientsComponent separationCholic acidLicorice acid
The invention relates to the technical field of the traditional Chinese medicine and in particular to the quality standard of a pharynx-clearing dropping pill which is made from the raw materials of a natural indigo, liquorice, myrobalam, menthol, borneol, artificial cow-bezoar, polyglycol 6000. The item of physicochemical identification in the former quality standard is deleted; the thin layer identification of the borneol and the menthol, the thin layer identification of indigotin or indirubin and cholic acid or deoxycholic acid and the item of mensuration of bilirubin content are revised; the thin layer identification of the myrobalam, the layer identification of the liquorice and the item of the mensuration and detection of glycyrrhizic acid content are added, and the quantitative index of the liquorice content in each pill can not be less than 32 microgrammes, which is calculated according to the glycyrrhizic acid (C42H62O16). The invention improves the controllability of the quality standard of the pharynx-clearing dropping pill and further ensures the internal quality of the product, and the invention has great meaning for promoting the product sale and guaranteeing the medication safeness for the patient.
Owner:津药达仁堂集团股份有限公司第六中药厂
NOVEL 7Beta-HYDROXYSTEROID DEHYDROGENASE MUTANTS AND PROCESS FOR THE PREPARATION OF URSODEOXYCHOLIC ACID
The invention relates to novel 7β-hydroxysteroid dehydrogenase mutants, to the sequences which encode these enzyme mutants, to processes for the preparation of the enzyme mutants and to their use in enzymatic reactions of cholic acid compounds, in particular in the preparation of ursodeoxycholic acid (UDCS). The invention also relates to novel processes for the synthesis of UDCS using the enzyme mutants; and to the preparation of UDCS using recombinant, multiply-modified microorganisms.
Owner:PHARMAZELL GMBH
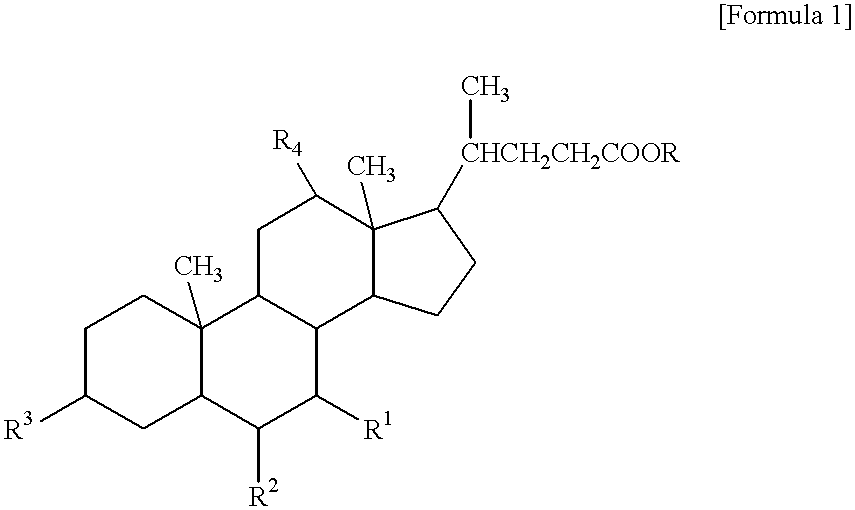
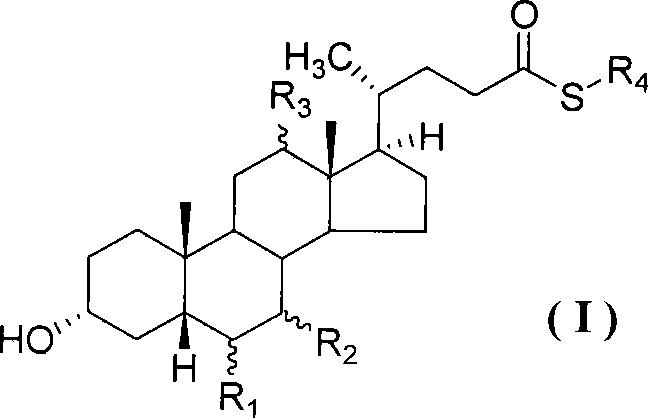
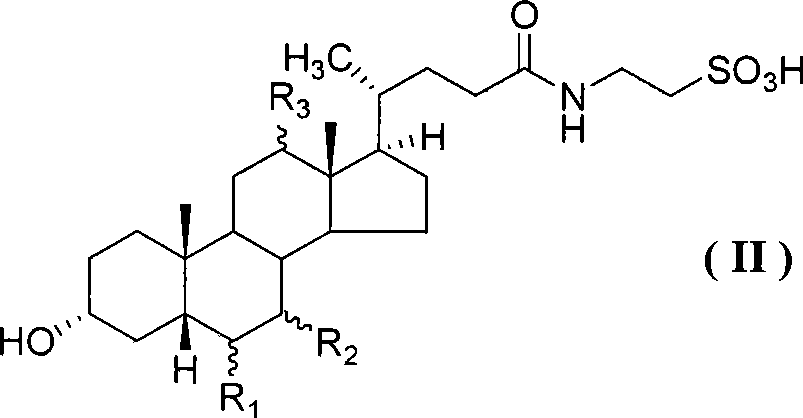
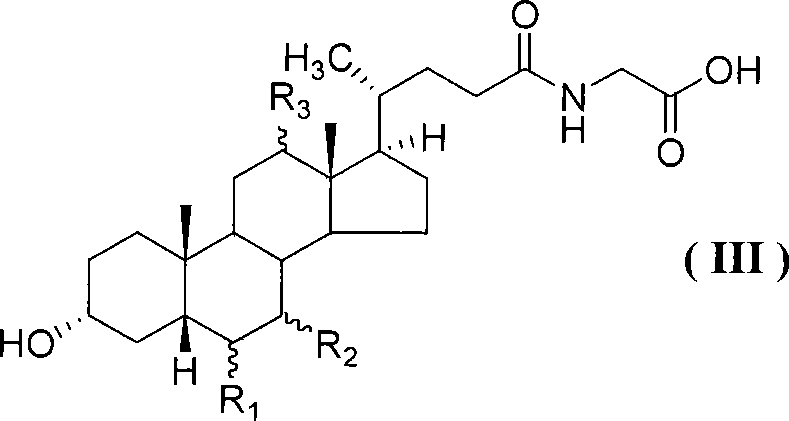
Compound for preparing cholic acid conjugate, preparation and use thereof
InactiveCN101503454AReduce pollutionRaw materials are cheap and easy to getOrganic active ingredientsMetabolism disorderCholeic AcidThioester
The invention discloses cholic acid compound activated thioester (1), also discloses s preparation method for the compound and the application thereof in the cholic acid conjugate therapeutic drug; (R 1, R2, and R3 in the formula express H, alpha-OH, beta-OH, =O ; R1, R2 and R3 can be same and can also be different; R4 presents 2- pyridil and 2- benzothiazolyl ).
Owner:SICHUAN UNIV
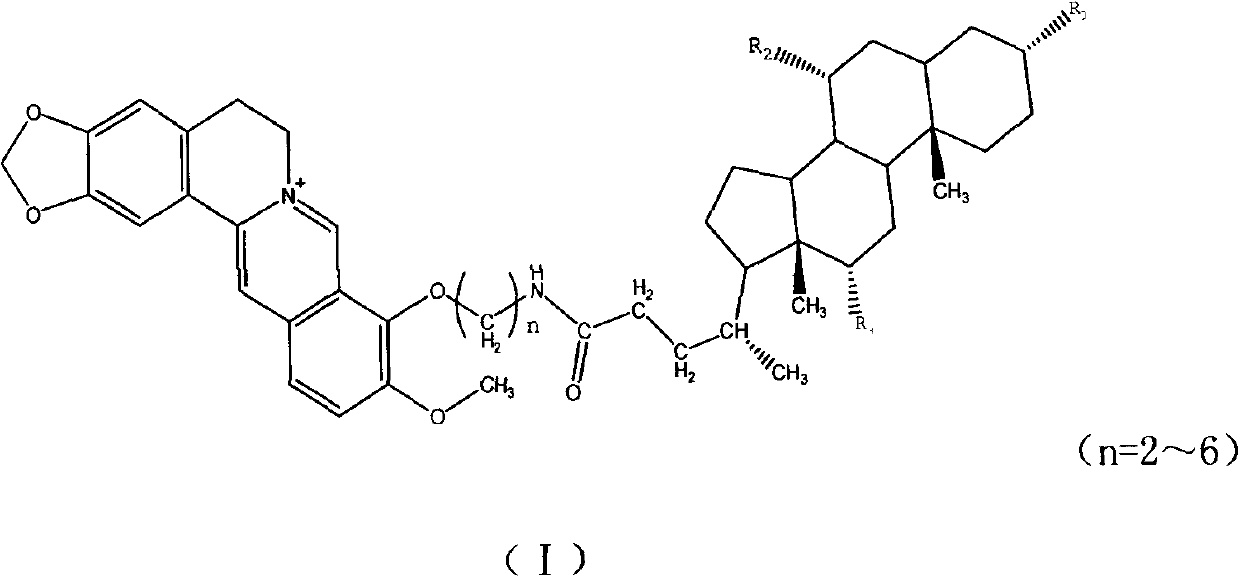
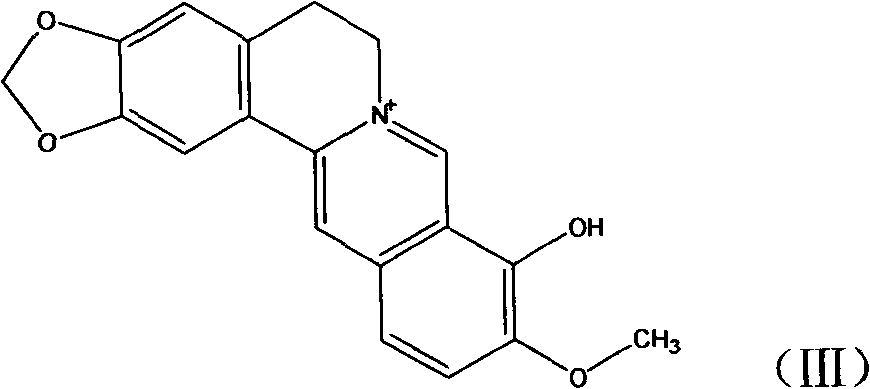
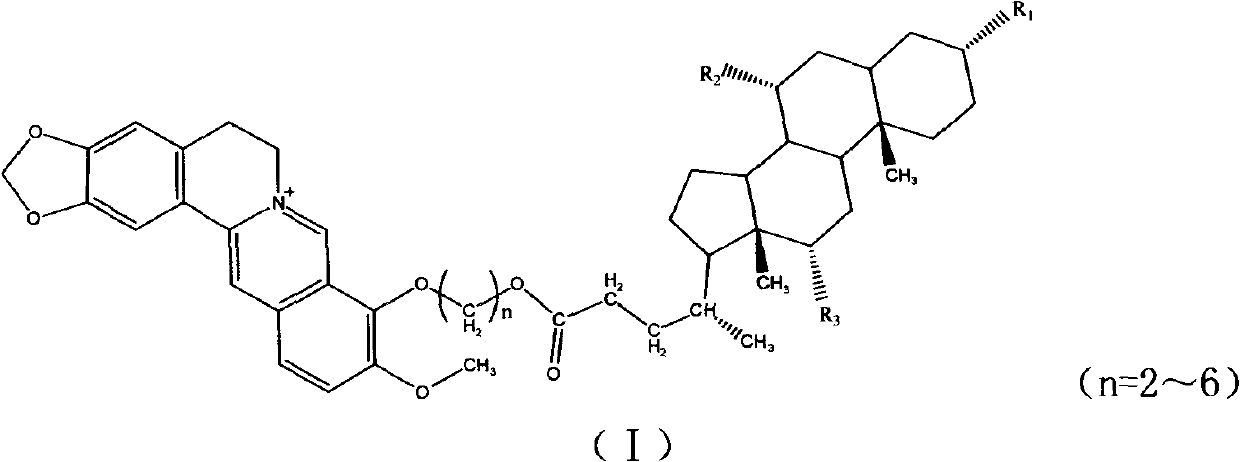
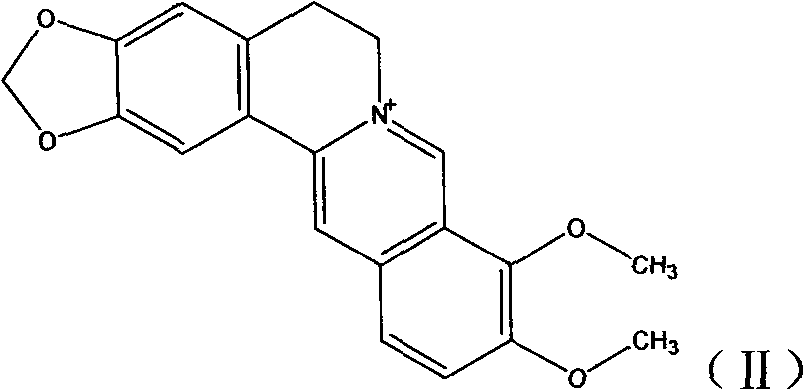
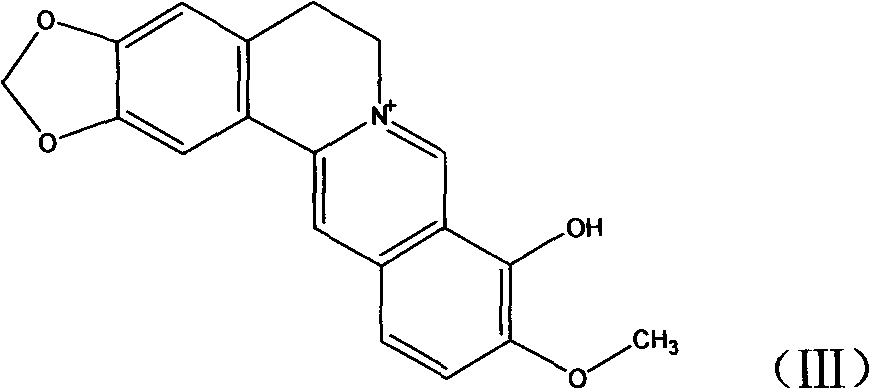
Novel berberine 9-position coupled cholic acid derivative and preparation method thereof
InactiveCN102225961AMaintain pharmacological activityGuaranteed stabilityOrganic active ingredientsSteroidsCholic acidBerberine
The invention relates to a novel berberine 9-position coupled cholic acid derivative as shown in a formula (I) and a preparation method thereof as well as application of the derivative in the aspect of being a medicament, especially treating tumour. In the formula (I), R1 is hydroxyl or carbonyl; and R2 and R3 are hydrogen, hydroxyl or carbonyl.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Novel 7alpha-hydroxysteroid dehydrogenase knockout mutants and use thereof
The invention relates to novel microbial 7alpha-hydroxysteroid dehydrogenase (7alpha-HSDH) knockout mutants and to the use thereof for producing other HSDHs having various functionalities, such as 3alpha-, 7beta- or 12alpha-HSDH, and to the use of thus-produced HSDH enzymes in enzymatic reactions of cholic acid compounds, and in particular for producing ursodeoxycholic acid (UDCS). The invention relates in particular to novel methods for synthesizing UDCS.
Owner:CELL PHARM CO LTD
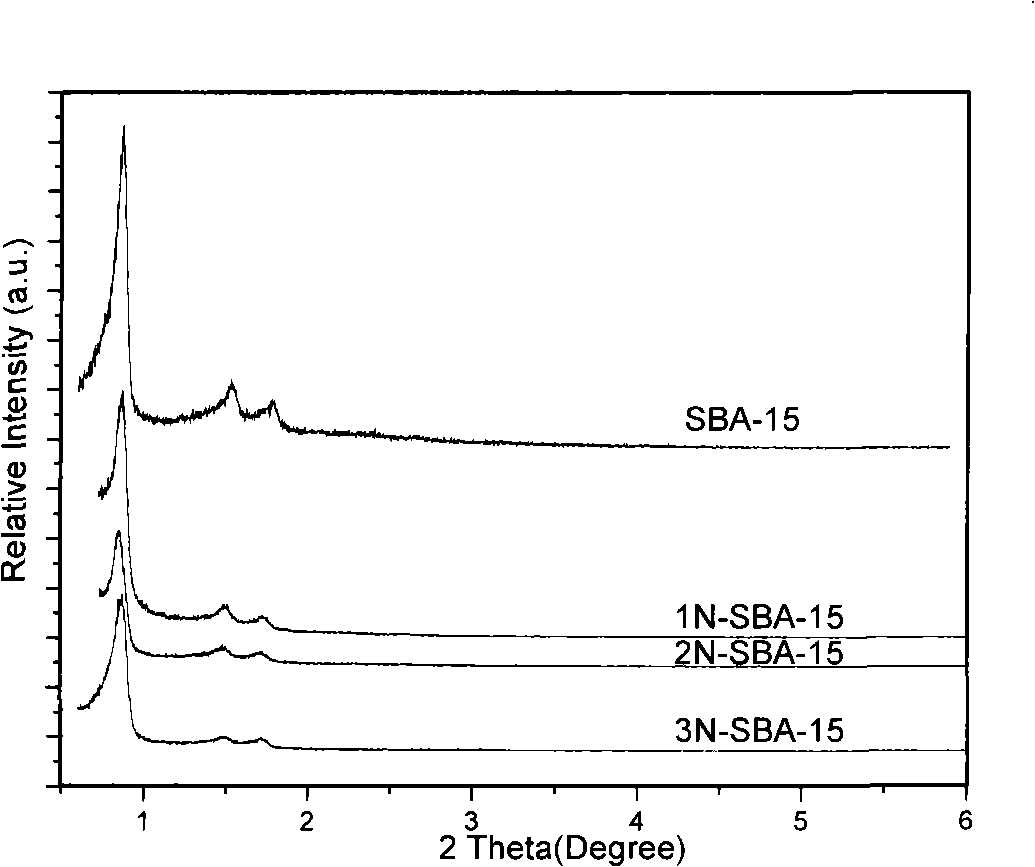
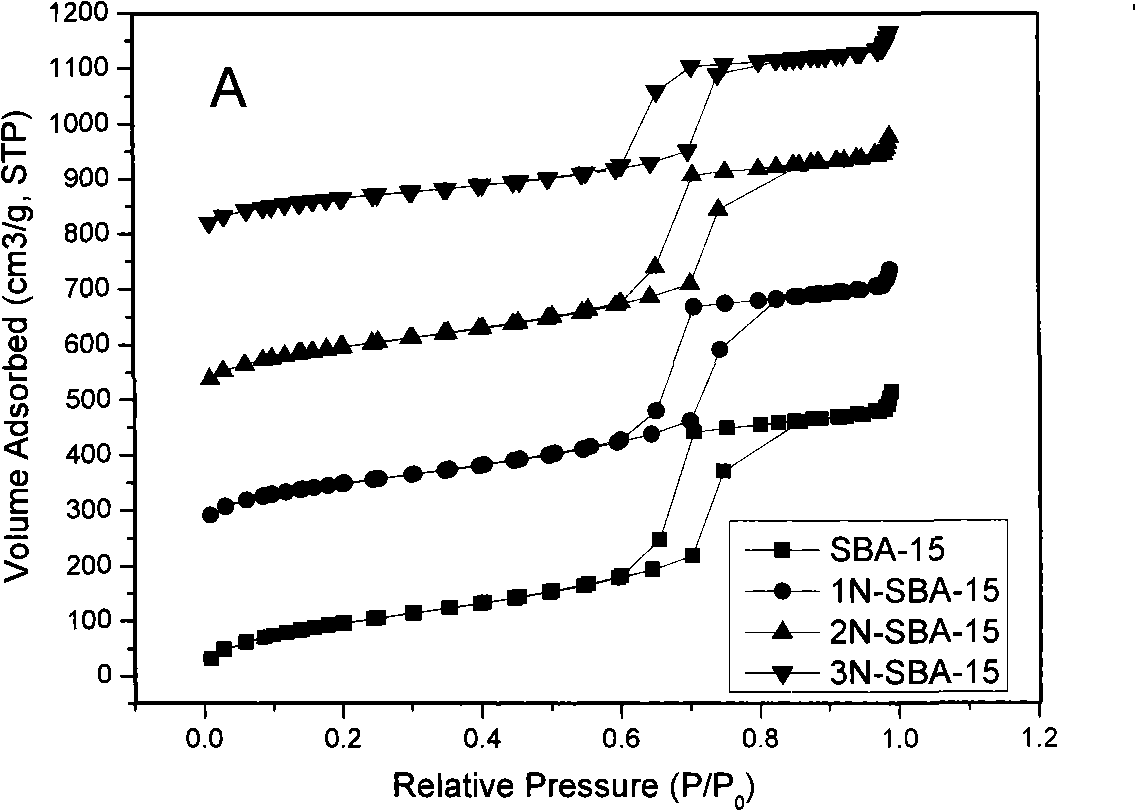
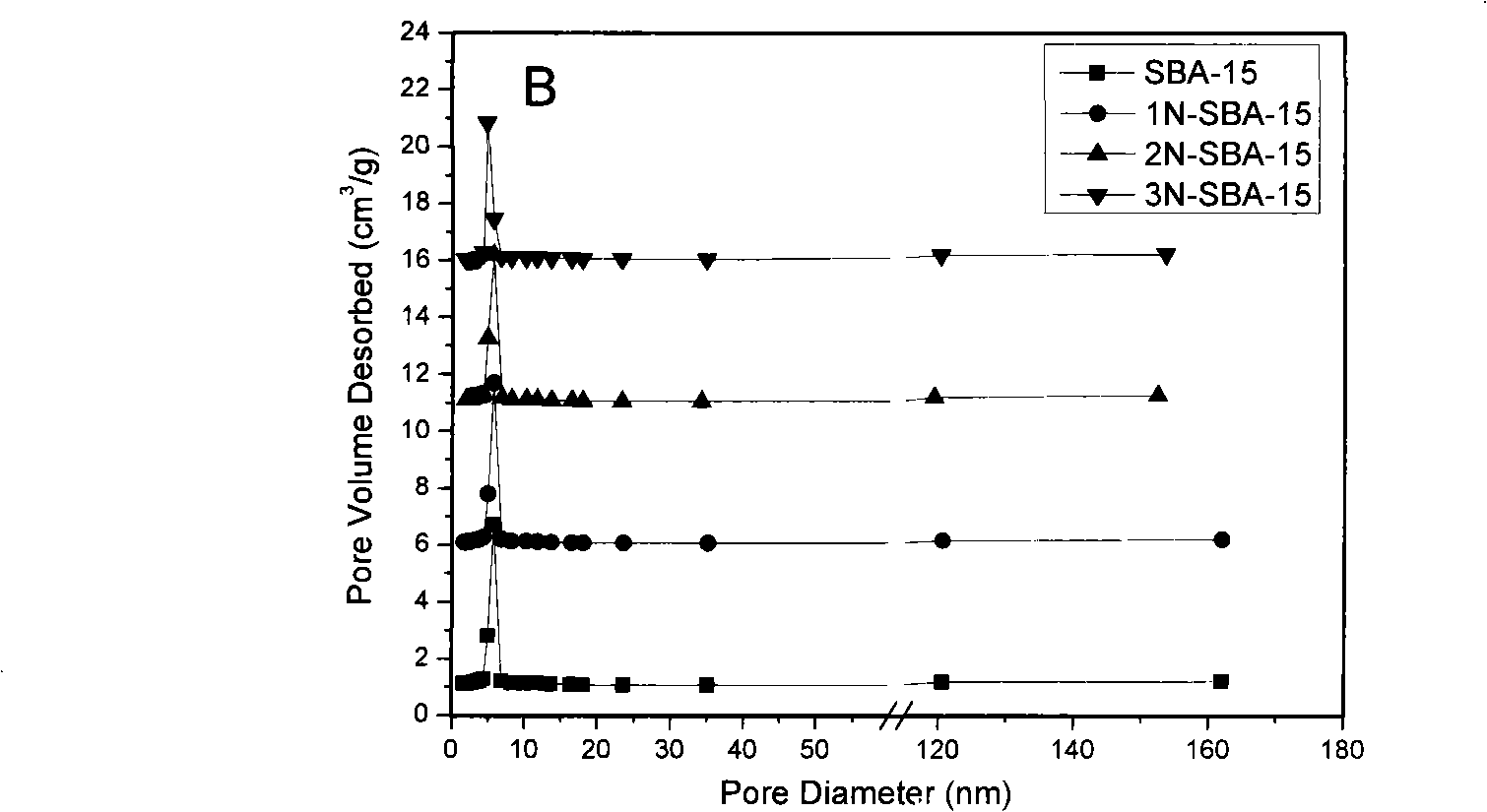
Mesoporous medical sorbent material
The invention relates to a mesoporous medical adsorption material, belonging to the field of mesporous material. The mesoporous adsorbent material of the invention has orderly mesoporous opening structure and composition of SiO2; amino modification of the surface of the material can be carried out. The adsorbent material of the invention can carry out the medical adsorption on various harmful matters such as bilirubin, cholic acid, uric acid, etc., and has good adsorption effect.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
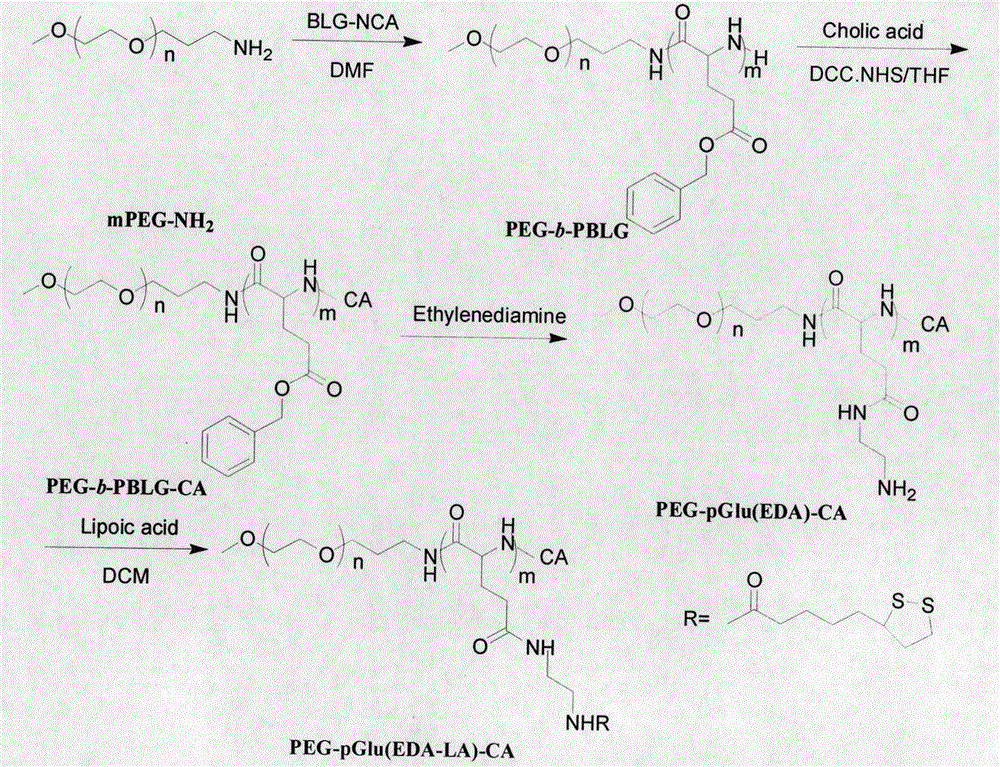
Synthesis method and use of cholic acid-modified polyamino acid block copolymer
ActiveCN105524271AReduction sensitiveImprove loading efficiencyPharmaceutical non-active ingredientsEmulsion deliveryTreatment effectCancer cell
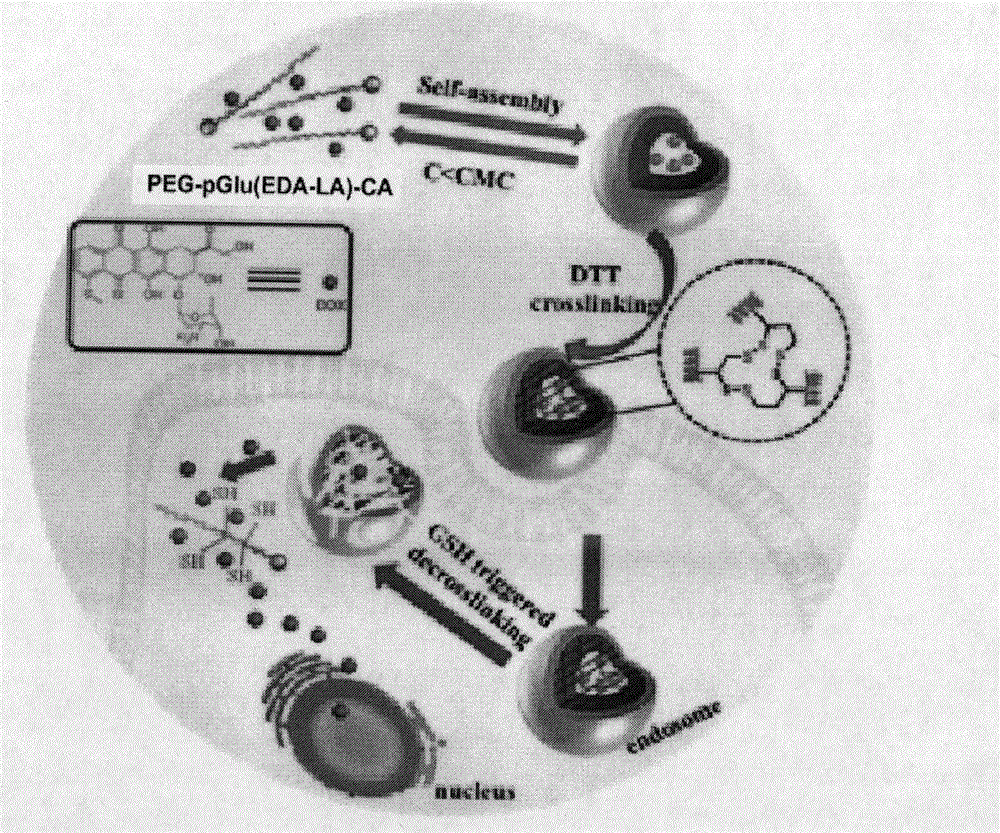
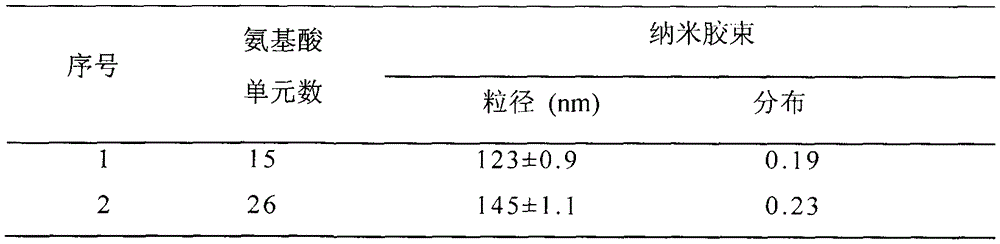
The invention discloses a synthesis method and use of a cholic acid-modified polyamino acid block copolymer. A hydrophilic chain of the block copolymer is polyethylene glycol, a hydrophobic chain of the block copolymer is polyamino acid, the tail end of the polyamino acid is modified through micromolecular cholic acid, the side chain of the polyamino acid is a lipoyl group, and lipoic acid and an amino group of hydrophobic polyamino acid undergo a reaction to produce an amido bond. Through crosslinking of a self-assembled nanometer micelle of the cholic acid-modified polyamino acid block copolymer, a stable crosslinked reduction-sensitive polymer nanometer micelle is obtained so that the nanometer micelle is not easily damaged in vitro and in blood and nanometer micelle-coated drug stability is guaranteed. When the nanometer micelle enters a cancer cell, the nanometer micelle can be fast decrosslinked and dissociated and the coated drug can be fast released and produce high efficiency treatment effects. The cholic acid-modified polyamino acid block copolymer solves the problems of early release of a drug in vivo, low carrying efficiency and a slow release rate in cells.
Owner:徐州康宇再生资源科技有限公司
Preparation and medical application of cholic acid berberine conjugate
InactiveCN105693805APrevent and treat cirrhosisPrevention and treatment of fatty liverOrganic active ingredientsMetabolism disorderCholic acidAcute hyperglycaemia
The invention provides a preparation method of a conjugate in the formula (I) and application thereof as medicine preventing or treating liver cirrhosis, adiposis hepatica, hyperlipidemia, hyperglycemia, obesity and hepatic fibrosis. Pharmacological experiments find that the conjugate has drug activity with various value. Specifically, the conjugate shows the functions of reducing blood sugar and low-density lipoprotein and improving the transaminase level in animal experiments. Besides, the invention further relates to a preparation, a preparation method and application of the conjugate.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Quality inspection method of traditional Chinese medicine composition twenty-five-ingredient lung disease preparation
ActiveCN102749401AGuarantee safe and effectiveAdd inspection itemsComponent separationDiseaseCholic acid
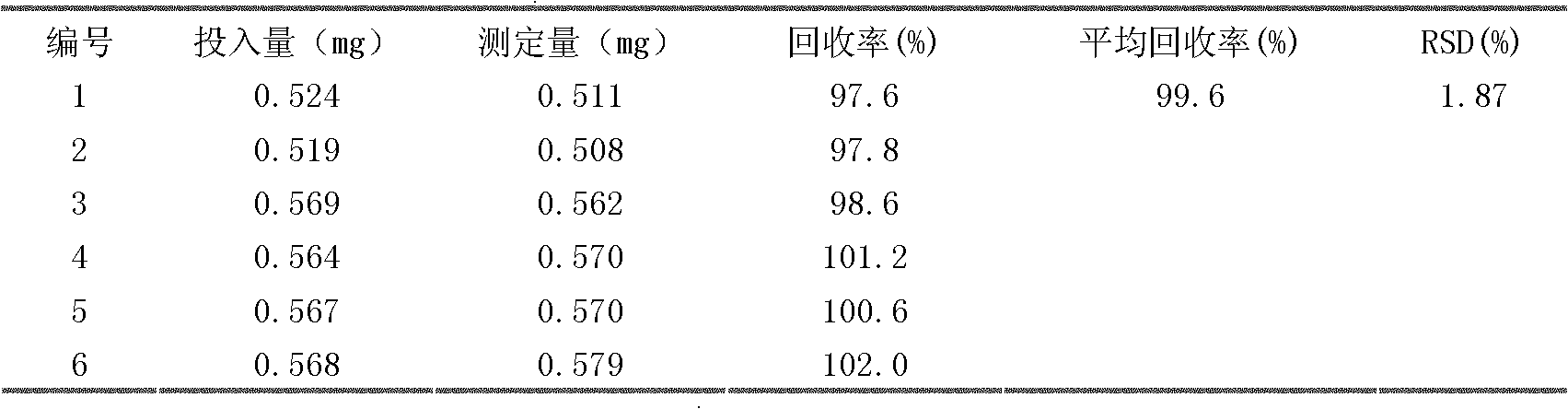
The invention discloses a quality inspection method of a traditional Chinese medicine composition twenty-five-ingredient lung disease capsule and a preparation thereof. On the basis of the primary standard, high-performance liquid chromatography (HPLC) is additionally used to perform limit test to aconitine in the twenty-five-ingredient lung disease capsule, and simultaneously the high-performance liquid chromatography (HPLC) is adopted to perform quantitative detection to active ingredients of hydroxysafflor yellow A, swertiamarin and cholic acid, as well as differentiation to fructus terminaliae billericae, sandalwood, kummel, Baxiaga and licorice root. The method improves the product quality, and ensures that the preparation can guarantee the drug use safety and the effectiveness while curing diseases.
Owner:JINHE TIBETAN MEDICINE
Adefovir dipivoxil ester colalin derivatives as well as preparation method and uses thereof
InactiveCN101182331AIncrease concentrationGood treatment effectOrganic active ingredientsGroup 5/15 element organic compoundsCholeic AcidStereochemistry
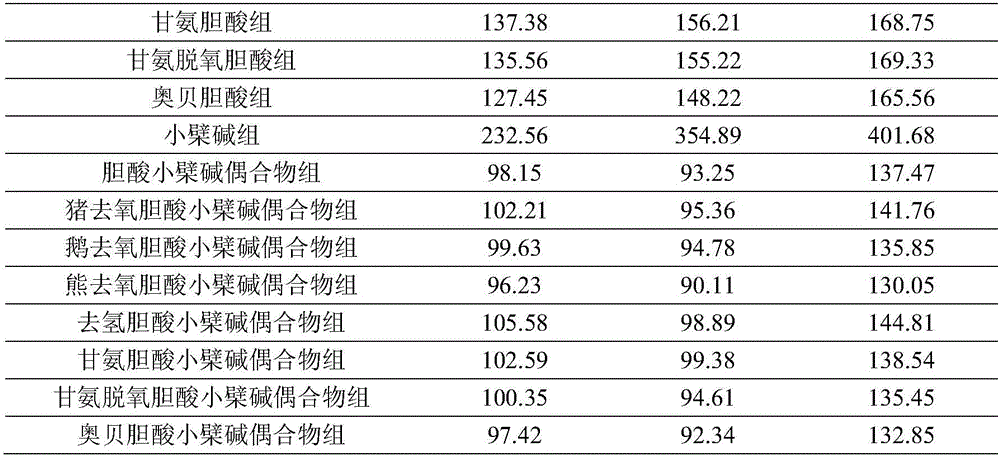
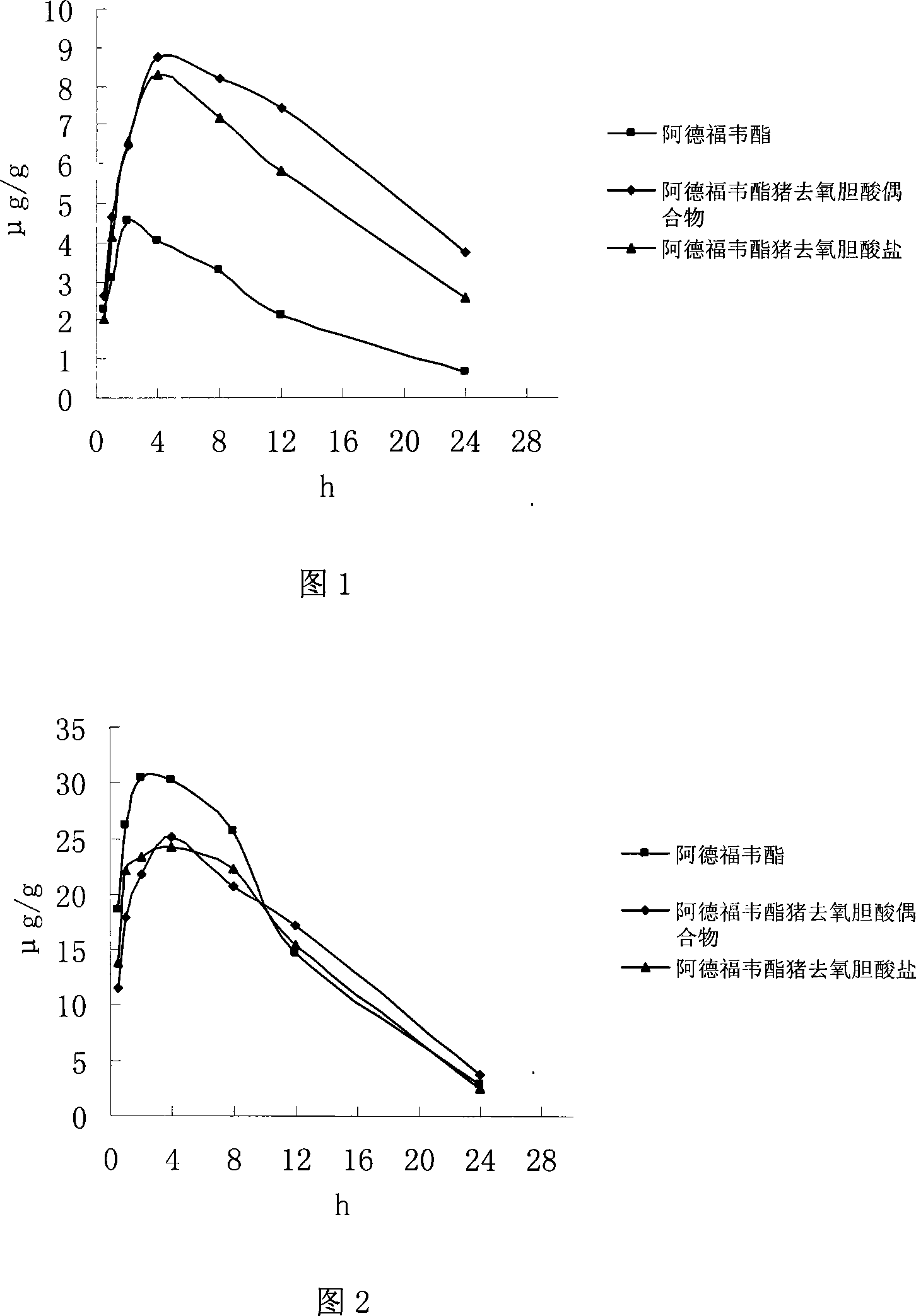
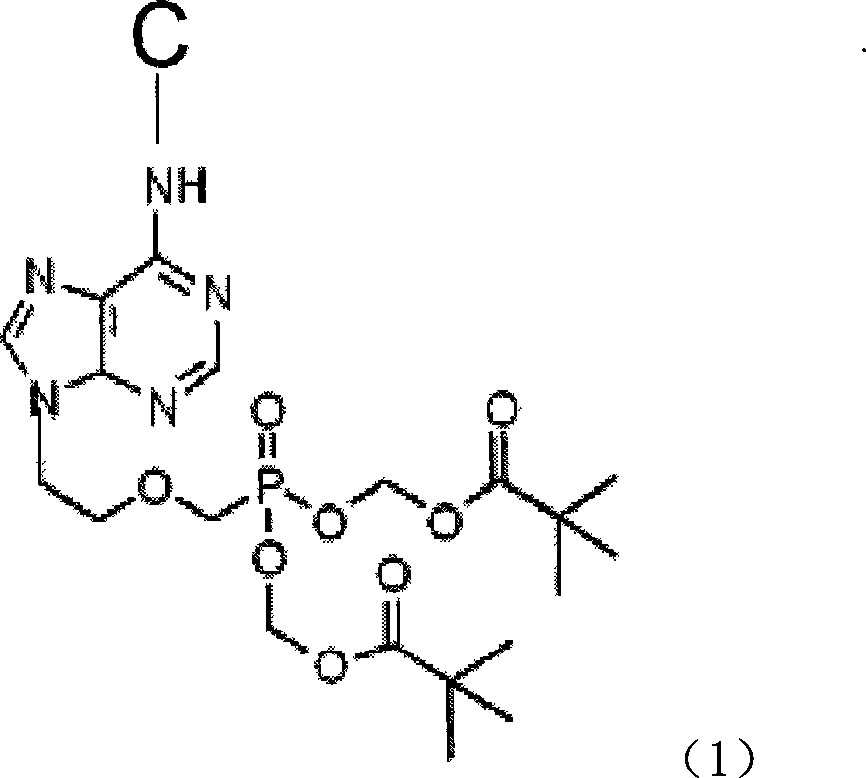
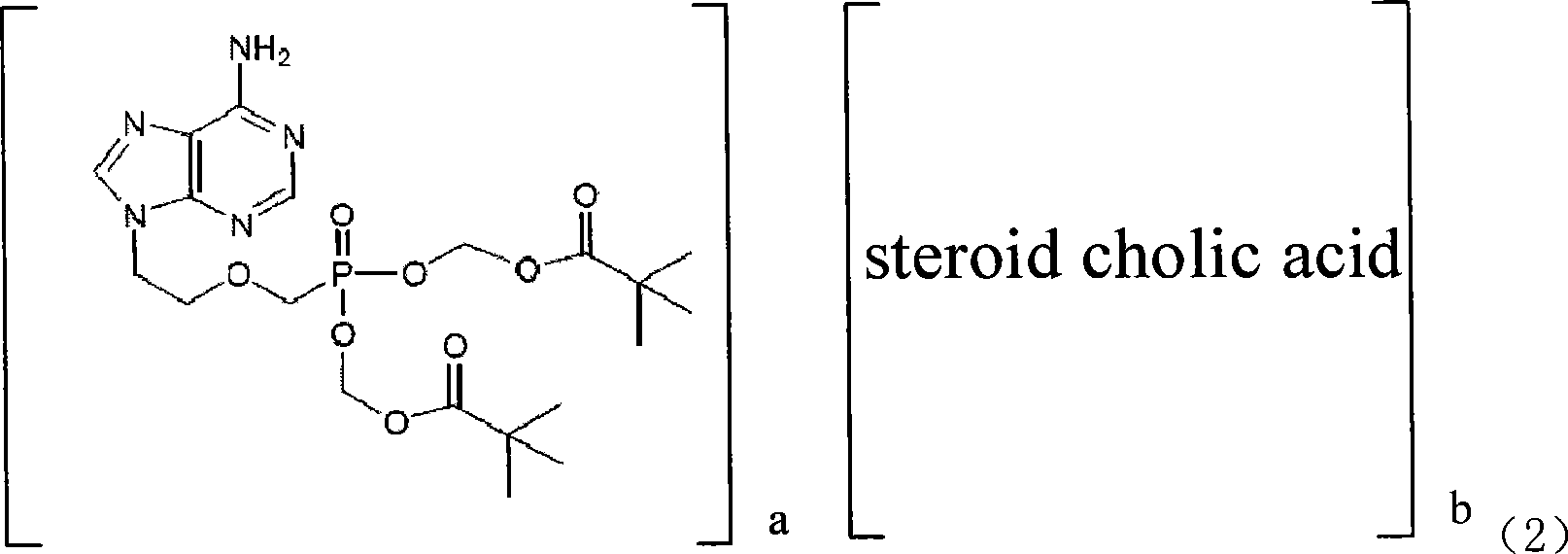
The present invention discloses an adefovir dipivoxil cholic acid class derivative and also relates to a preparation method and applications thereof at the medical aspect. The adefovir dipivoxil cholic acid class derivative is a hepatic targeting precursor drug of adefovir; compared with the adefovir dipivoxil, the adefovir dipivoxil cholic acid class derivative can improve the concentration of the adefovir in liver after being taken orally, and the adefovir dipivoxil cholic acid class derivative can be used as a preferential drug for the treatment of resisting virus and especially resisting hepatitis virus.
Owner:CHINA PHARM UNIV
Method for detecting quality of An'erning granules
The invention discloses a method for detecting the quality of An'erning granules. The method comprises a method for identifying cholic acid and bergenin and a method for determining content of glycyrrhizic acid, the bergenin and bilirubin. Experimental study proves that spots with different colors are displayed at a position, which corresponds to a color spectrum of a reference substance, in the color spectrum of a sample by the identification method in the quality detection method; and negative control is not interfered. Results show that the content determination method is used for determining the content of the glycyrrhizic acid and the bergenin in the An'erning granules, has a good linear relation, and high repeatability and stability, and can control the quality of An' erning granules accurately and strictly; and moreover, the accuracy of recovery tests is high.
Owner:JINHE TIBETAN MEDICINE
Reversibly crosslinked micelle systems
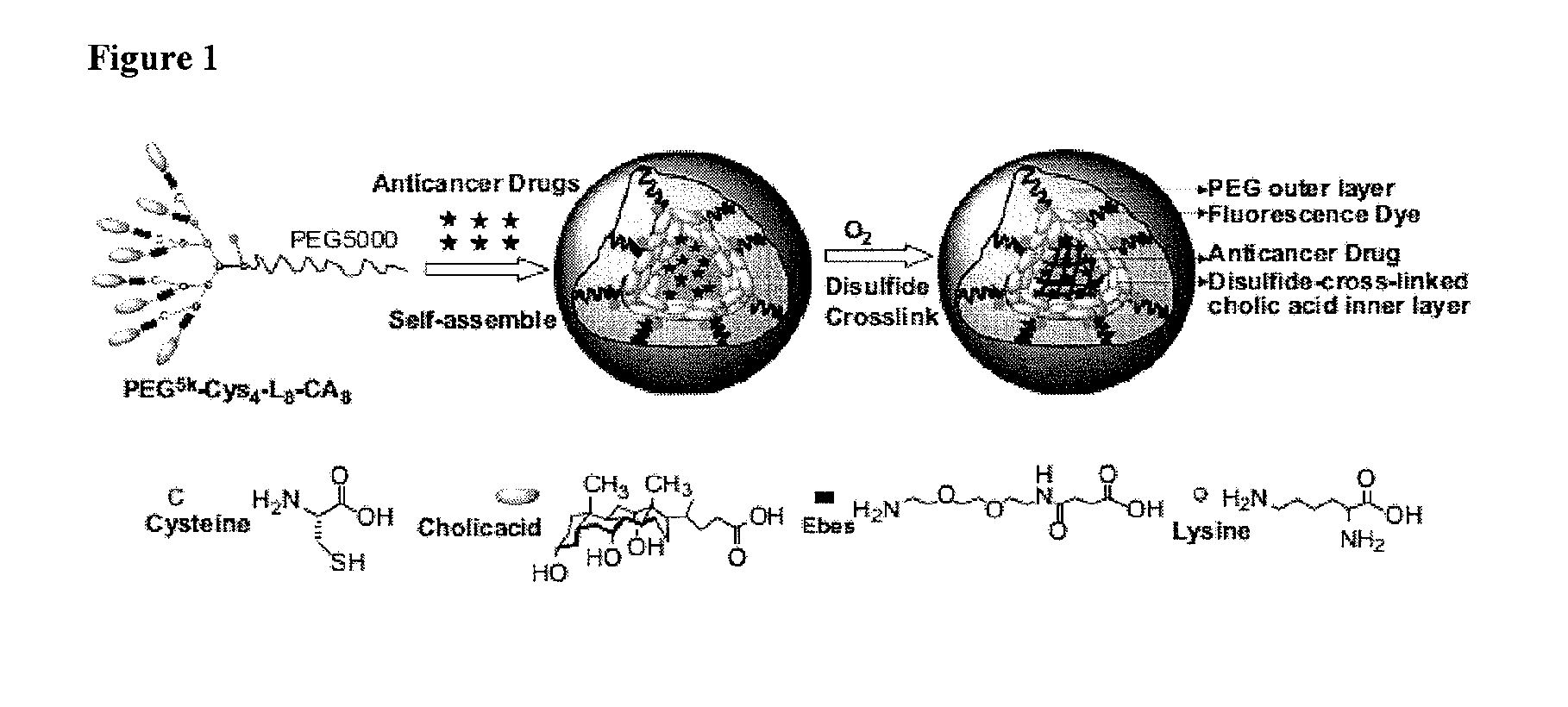
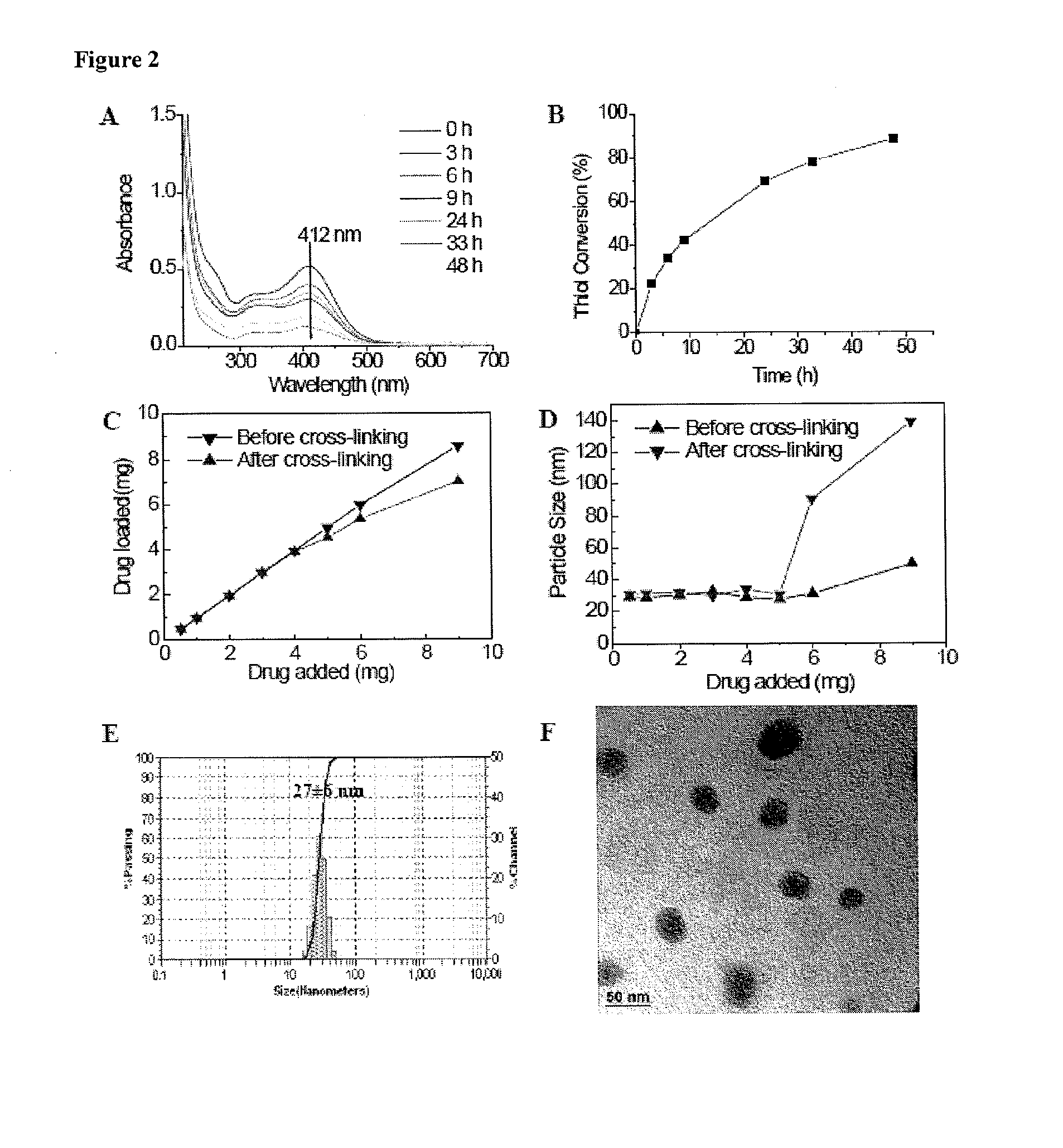
The present invention provides amphiphilic telodendrimers that aggregate to form nanocarriers characterized by a hydrophobic core and a hydrophilic exterior. The nanocarrier core may include amphiphilic functionality such as cholic acid or cholic acid derivatives, and the exterior may include branched or linear poly(ethylene glycol) segments. Nanocarrier cargo such as hydrophobic drugs and other materials may be sequester in the core via non-covalent means or may be covalently bound to the telodendrimer building blocks. Telodendrimer structure may be tailored to alter loading properties, interactions with materials such as biological membranes, and other characteristics.
Owner:RGT UNIV OF CALIFORNIA
Glycated albumin enzymatic detection kit and detection method thereof
The invention relates to a glycated albumin (GA) enzymatic detection kit, which comprises a GA reagent 1 and a GA reagent 2. The GA reagent 1 comprises tris(hydroxymethyl)aminomethane, aminoantipyrine (4-AAP), protease K, calcium acetate (CaAc2), potassium ferrocyanide trihydrate (K4Fe(CN)6.3H2O), copper acetate (CuAc2) and Cholic acid. The GA reagent 2 includes tris(hydroxymethyl)aminomethane, N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methylaniline sodium salt (TOOS), fructosaminase, Triton X-100 and peroxidase. The invention aims to provide the glycated albumin enzymatic detection kit and its detection method with the characteristics of strong specificity, high sensitivity, short time, simple operation mode, and accurate and reliable detection result.
Owner:BEIJING HOMA BIOLOGICAL ENG
New derivatives of berberine coupled with cholic acid at ninth position through ester bond and preparation methods thereof
InactiveCN102229636AMaintain pharmacological activityGuaranteed stabilityOrganic active ingredientsSteroidsCholic acidBerberine
The invention relates to new derivatives of berberine coupled with cholic acid at the ninth position through an ester bond with a general formula (I) and preparation methods thereof. The invention also relates to the preparation methods of compounds of the present invention, and an application of the compounds as drugs especially as the drugs for tumor treatment. R1 in the formula (I) is hydroxy and / or carbonyl; and R2 / R3 is hydrogen, hydroxy and / or carbonyl.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Methods for diagnosis and intervention of hepatic disorders
ActiveUS8778299B2In-vivo radioactive preparationsMicrobiological testing/measurementCholic acidChronic hepatitis
Owner:UNIV OF COLORADO THE REGENTS OF
Cationic lipoid plastid and preparation method thereof
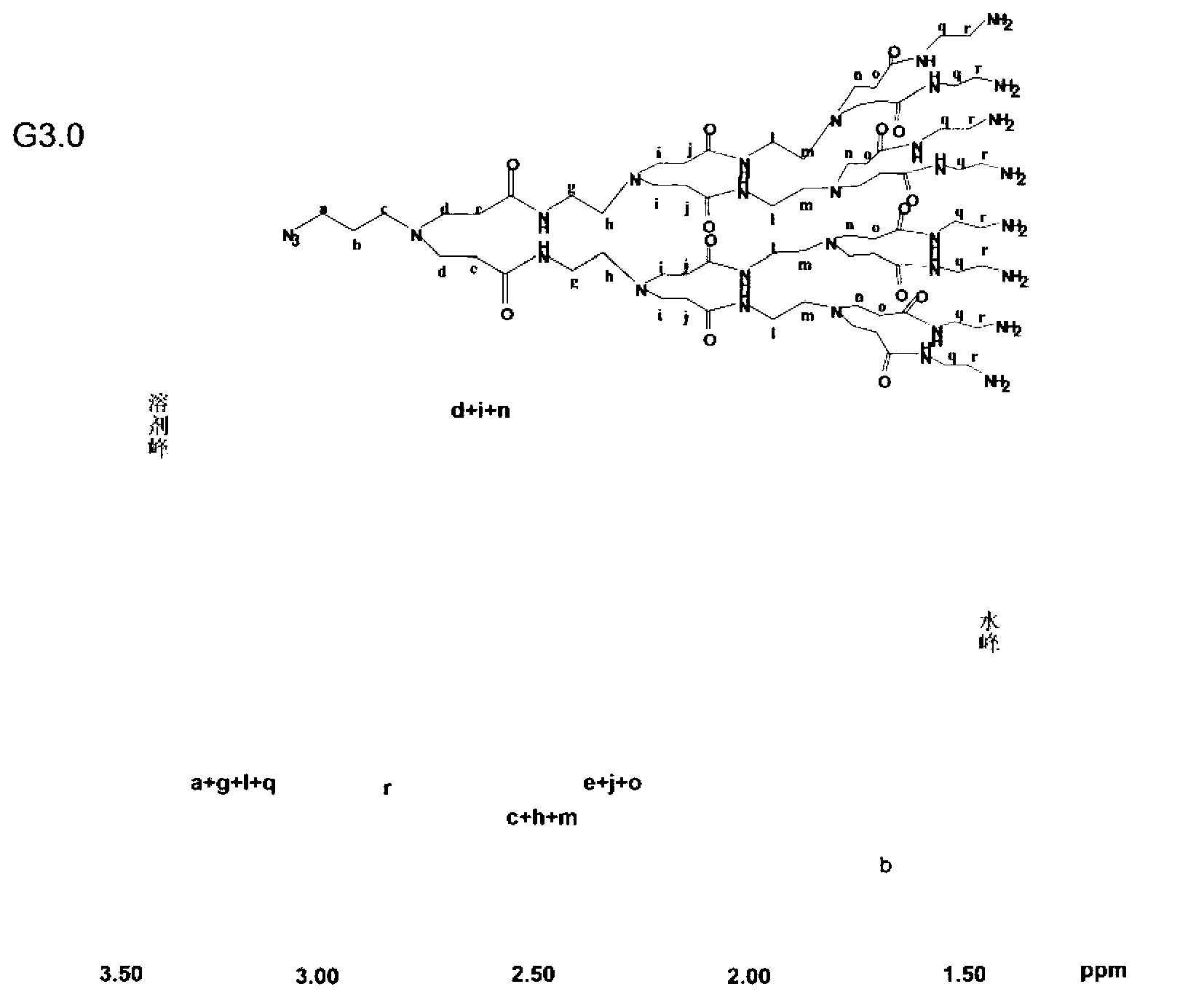
The invention provides a cationic lipoid plastid presented as formula I, wherein R is a substituent presented as formula II, III, IV and V, R' is C6-C40 of alkyl, C6-C40 of substituted alkyl, a cholesterol group or a cholic acid group. The novel cationic lipoid plastid takes PAMAM as a hydrophilic head and takes two hydrophobic chain sections as a hydrophobic tail, is good in biocompatibility and degradable, and provided with excellent properties of the PAMAM and lipoid plastid simultaneously. The invention further provides a preparation method of the cationic lipoid plastid presented as formula I. The method is high in generality, a plurality of compounds in similar structures can be obtained through the method, and a methodology platform is provided for studying the structure property relations of the compounds.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Spherical silicon dioxide hollow material and preparation method thereof
InactiveCN101792149ARaw materials are readily available and non-toxicSimple and fast operationNanostructure manufactureSilicaSilicon dioxideRaw material
The invention relates to a spherical silicon dioxide hollow material and a preparation method thereof. The spherical silicon dioxide hollow material is prepared from cholic acid and silicon source serving as raw materials in a molar ratio of 1: 2-6. The preparation method comprises the following steps: dissolving the cholic acid into aqueous solution of ammonia to form alkali aqueous solution of cholic acid ammonium salt, reacting the alkali aqueous solution with the silicon source with stirring, standing and ageing the solution, filtering a reaction product, and flushing and drying the product. The silicon source is a mixture of 3-aminopropyltriethoxysilane and ethyl orthosilicate in a ratio of 1: 1-5. The grain diameter of the spherical silicon dioxide hollow material is 100 to 1,000 nanometers, and the wall thickness is 50 nanometers. A micro-molecular biological surfactant is used as a template, an organic solvent is not used in the reaction, a template agent does not need to be further removed, the raw materials are easily obtained and are nontoxic, the operation is simple and convenient, and the conditions are mild. The silicon dioxide hollow material prepared by the method is particularly applicable in biomedicine related fields of medicament delivery, active guest molecule protection and the like.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com