Compound for preparing cholic acid conjugate, preparation and use thereof
A technology of compounds and conjugates, applied in the preparation of cholic acid conjugates therapeutic drugs, in the field of active thioesters of cholic acid compounds, can solve harsh reaction conditions, "three wastes" discharge, low total yield, etc. problems, to achieve the effect of mild reaction conditions, less pollution of "three wastes" and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
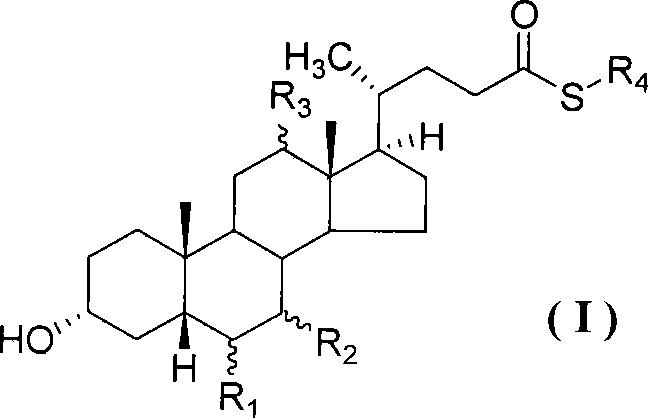
[0038] Example 1 Preparation of ursodeoxycholic acid-2-benzothiazole thioester
[0039] Add 15.7 grams (0.04mol) of ursodeoxycholic acid, 8.0 grams (0.048mol) of triethyl phosphite, 16.0 grams (0.048mol) of 2,2'-dithiodibenzothiazole and ethyl acetate in the reaction flask 100 ml, stirred at room temperature for 1 hour, added dropwise a solution of 7.0 ml of triethylamine dissolved in 20 ml of ethyl acetate, heated up to 35-40°C for 2.5 hours, cooled to room temperature, and evaporated under reduced pressure Remove the solvent and purify by column chromatography (eluent: chloroform: acetone: acetic acid = 20:1:1 (v / v)), to obtain the light yellow oily liquid 19.3 of ursodeoxycholic acid-2-benzothiazole thioester grams, yield 89.0%. 1 HNMR (CDCl 3 )δ: 8.03 (dd, J 1 = 0.8Hz,J 2 =8.0Hz, 1H, Ar-H 4 ), 7.91 (dd, J 1 = 0.8Hz,J 2 =7.2Hz, 1H, Ar-H 7 ), 7.52~7.41 (m, 2H, Ar-H 5 , Ar-H 6 ), 3.67~3.60 (m, 2H, C 7 -H,C 3 -H), 2.87~2.80(m, 1H, C 23 -H), 2.78~2.65(m, 1H, C 23 ...
Embodiment 2
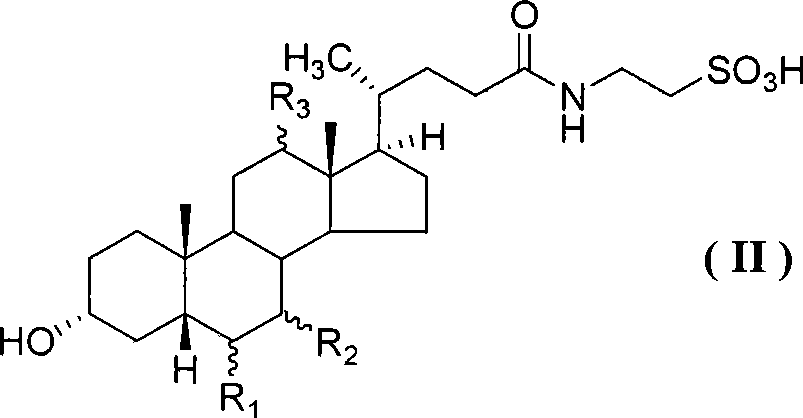
[0040] Example 2 Preparation of Tauroursodeoxycholic Acid
[0041] Add 3.0 g (0.024 mol) of taurine, 0.88 g (0.024 mol) of sodium hydroxide and 40 ml of water into the reaction flask, stir evenly, cool in an ice-water bath to 0-10°C, add ursodeoxycholic acid-2 dropwise - A solution of 10.8 grams (0.02 mol) of benzothiazole thioester dissolved in 30 milliliters of tetrahydrofuran was stirred and reacted at room temperature for 8 hours. × 20 ml extraction, the resulting water layer was concentrated under reduced pressure to leave about 20 ml, adjusted to pH 1-2 with concentrated hydrochloric acid, added a small amount of seed crystals, stirred at 0-5°C for 2 hours, filtered with suction, washed the filter cake with a small amount of acetone, and then Recrystallize with acetone-water (12:1) to obtain 7.5 grams of tauroursodeoxycholic acid white powder solid, mp144~145°C, (c=2.0, EtOH), yield 78.6%. Purity 99.2% (HPLC normalization method). 1 HNMR (400MHz, D 2 O) δ: 3.65 ~ 3....
Embodiment 3
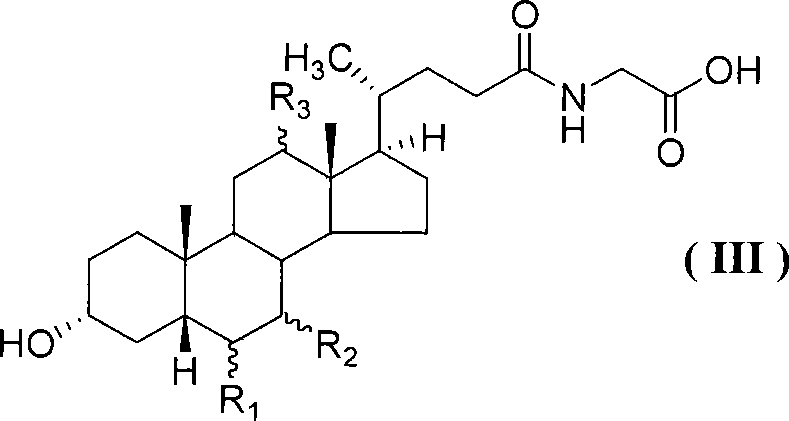
[0042] Example 3 Preparation of Glycoursodeoxycholic Acid
[0043] Add 3.35 g (0.024 mol) of glycine ethyl ester hydrochloride, 3.34 ml (0.024 mol) of triethylamine and 35 ml of ethyl acetate into the reaction flask, stir well, put in an ice-water bath to cool to 0-10°C, add dropwise 10.8 g (0.02 mol) of ursodeoxycholic acid-2-benzothiazole thioester was dissolved in 30 ml of ethyl acetate, stirred at room temperature and reacted overnight, after the reaction was completed, the insoluble matter was filtered off, and the ethyl acetate solution was sequentially used with 5% Wash with 36 ml of NaOH aqueous solution, 30 ml of 10% aqueous hydrochloric acid solution, 30 ml of saturated NaCl aqueous solution, anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain the crude ursodeoxycholoylglycine ethyl ester conjugate. The obtained crude product was dissolved in a mixed solution of 0.8 g (0.02 mol) of sodium hydroxide, 20 ml of water and 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com