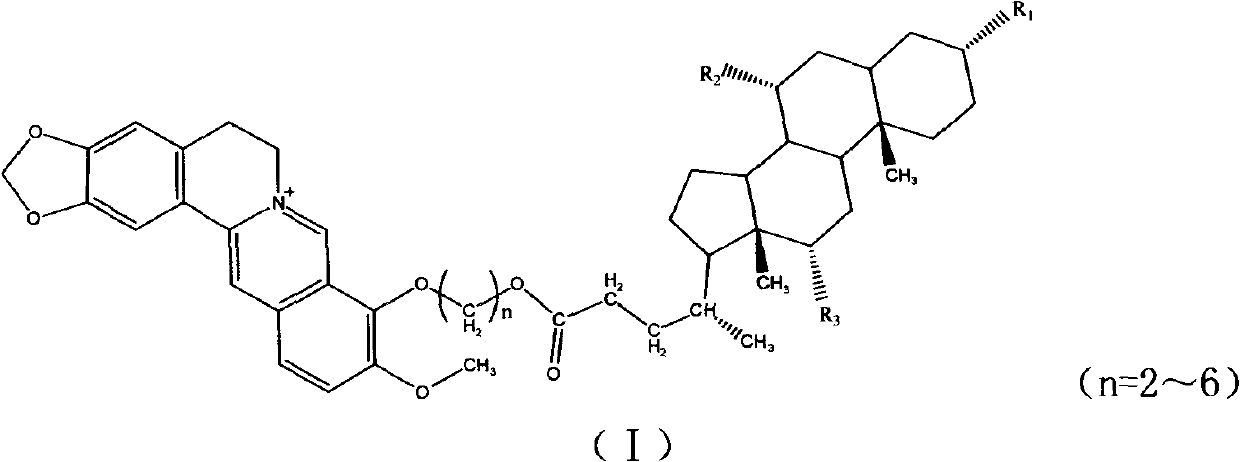
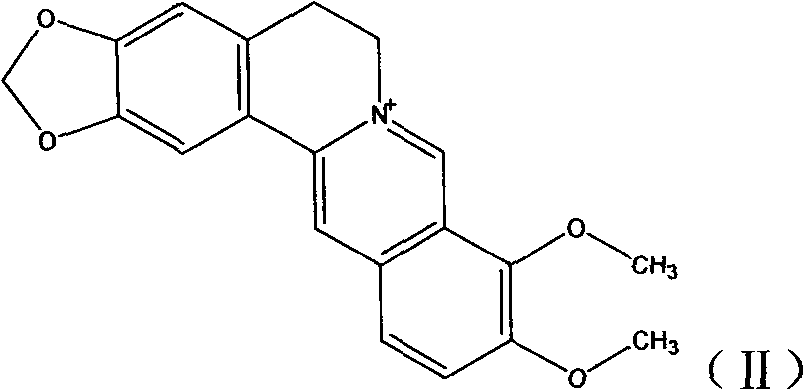
New derivatives of berberine coupled with cholic acid at ninth position through ester bond and preparation methods thereof
A derivative, berberine technology, applied in the field of preparation of berberine derivatives, can solve the problems of low bioavailability, poor targeting, poor fat solubility, etc., achieve good fat solubility, maintain pharmacological activity, increase biological The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of cholic acid-9-propoxy-berberine:
[0033] (1) Synthesis of Berberine
[0034] Add 744mg (2mmol) of berberine into a 250mL round bottom flask, heat at 195-210°C for 30min at a vacuum of 20-30mmHg, the yellow solid gradually turns dark red, cool to room temperature in a vacuum desiccator, and perform silica gel column chromatography Purified to obtain a dark red powder with a yield of 73%, molecular weight: 322, and the structure as follows:
[0035]
[0036] (2) Cholic acid-3-bromopropyl ester
[0037] Add 204 mg (0.5 mmol) of cholic acid into a 25 mL round bottom flask, add 1 mL of DMF to dissolve, add 200 μL of 1,3-dibromopropane, add K 2 CO 3 276mg (2mmol), TLC followed the reaction, after the reaction was complete, vacuum filtration, 50mL of chloroform was added to the filtrate, followed by 50mL of distilled water, 50mL of saturated NaHCO 3 Solution extraction 3 times, anhydrous MgSO 4 Dry, filter under reduced pressure to remo...
Embodiment 2
[0042] Embodiment 2: the synthesis of dehydrocholic acid-9-propoxyberberine: (B2)
[0043] (1) Synthesis of Berberine
[0044] Same as (1) in Example 1.
[0045] (2) Dehydrocholic acid-3-bromopropyl ester
[0046] Add 201 mg (0.5 mmol) of dehydrocholic acid into a 25 mL round bottom flask, add 1 mL of DMF to dissolve, add 200 μL of 1,3-dibromopropane, add K 2 CO 3 276mg (2mmol), TLC followed the reaction, after the reaction was complete, vacuum filtration, 50mL of chloroform was added to the filtrate, followed by 50mL of distilled water, 50mL of saturated NaHCO 3 Solution extraction 3 times, anhydrous MgSO 4 Dry, filter under reduced pressure to remove MgSO 4 Concentrated under reduced pressure, separated and purified by silica gel column chromatography to obtain a white powder with a yield of 95%; molecular weight: 522, and the structure is as follows:
[0047]
[0048] (3) Synthesis of dehydrocholic acid-9-propoxyberberine: (B2)
[0049] Add 161mg (0.5mmol) of berb...
Embodiment 3
[0051] Embodiment 3: the synthesis of deoxycholic acid-9-propoxyberberine: (B3)
[0052] (1) Synthesis of Berberine
[0053] Same as (1) in Example 1.
[0054] (2) Deoxycholic acid-3-bromopropyl ester
[0055] Add 201 mg (0.5 mmol) of dehydrocholic acid into a 25 mL round bottom flask, add 1 mL of DMF to dissolve, add 200 μL of 1,3-dibromopropane, add K 2 CO 3 276mg (2mmol), TLC followed the reaction, after the reaction was complete, vacuum filtration, 50mL of chloroform was added to the filtrate, followed by 50mL of distilled water, 50mL of saturated NaHCO 3 Solution extraction 3 times, anhydrous MgSO 4 Dry, filter under reduced pressure to remove MgSO 4 Concentrated under reduced pressure, separated and purified by silica gel column chromatography to obtain a white powder with a yield of 85%; molecular weight: 512, and the structure is as follows:
[0056]
[0057] (3) Synthesis of deoxycholic acid-9-propoxyberberine: (B3)
[0058] Add 161mg (0.5mmol) of berberine in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com