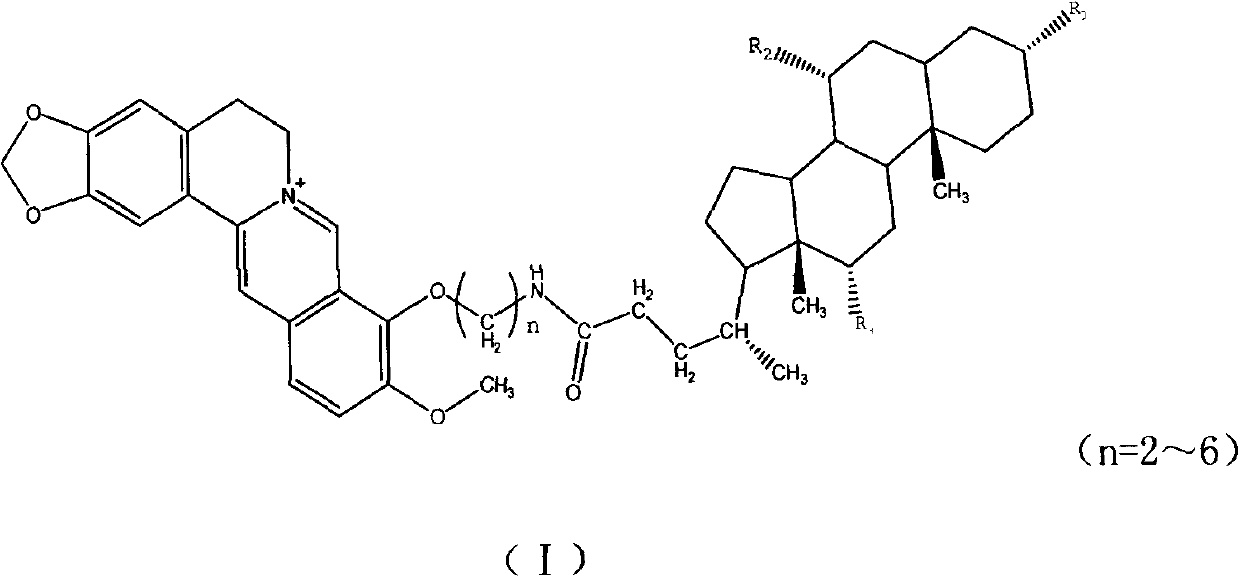
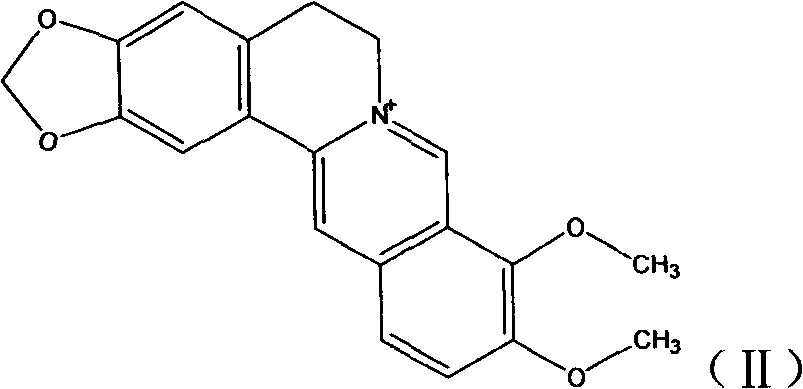
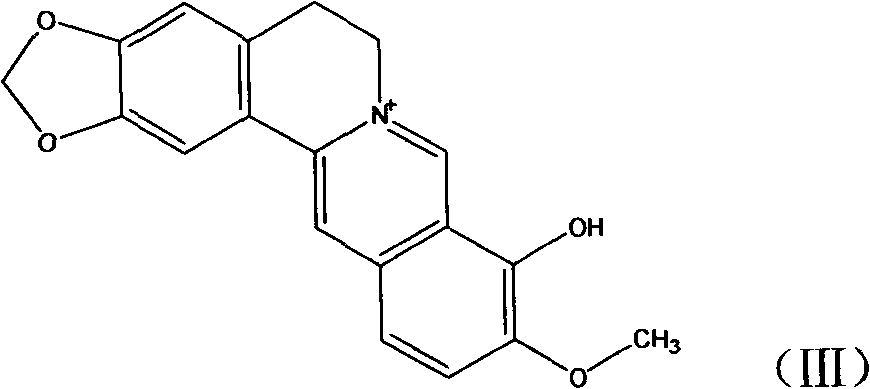
Novel berberine 9-position coupled cholic acid derivative and preparation method thereof
A derivative, berberine technology, applied in the field of preparation of berberine derivatives, can solve the problems of low bioavailability, poor targeting, poor fat solubility, etc., achieve good fat solubility, maintain pharmacological activity, increase biological The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of cholic acid-9-O-propionamide berberine: (A1)
[0045] (1) Synthesis of Berberine
[0046] Add 744mg (2mmol) of berberine into a 250mL round bottom flask, heat at 195-210°C for 30min at a vacuum of 20-30mmHg, the yellow solid gradually turns dark red, cool to room temperature in a vacuum desiccator, and perform silica gel column chromatography Purified to obtain a dark red powder with a yield of 73%, molecular weight: 322, and the structure as follows:
[0047]
[0048] (2) Synthesis of 9-O-3-bromo-propyl berberine
[0049] Add 161mg (0.5mmol) of berbererythrine into a 25mL round bottom flask, add 3mL of DMF to dissolve, heat at 60°C, add 500μL of 1,3-dibromopropane, the dark red solution gradually turns brownish yellow, follow the reaction by TLC, after the reaction is complete Add 10 mL of anhydrous diethyl ether, a yellow solid is precipitated, filtered under reduced pressure, and purified by silica gel column chromatography to obta...
Embodiment 2
[0060] Embodiment 2: the synthesis of dehydrocholic acid-9-O-propionamide berberine: (A2)
[0061] (1) Synthesis of Berberine
[0062] Same as (1) in Example 1.
[0063] (2) Synthesis of 9-O-3-bromopropyl berberine
[0064] Same as (2) in Example 1.
[0065] (3) Synthesis of 9-O-3-aminopropyl berberine:
[0066] Same as (3) in Example 1.
[0067] (4) Synthesis of dehydrocholic acid activated ester:
[0068] Add 201 mg (0.5 mmol) of dehydrocholic acid to a 50 mL round bottom flask, add 10 mL of dichloromethane, add 115 mg of N-hydroxysuccinimide, and add 1-ethyl-3-(3-dimethylaminopropyl Base) carbodiimide 766mg (4mmol) dichloromethane solution 10mL, follow the reaction by TLC. After the reaction was complete, 30 mL of chloroform was added to the reaction liquid, followed by 50 mL of saturated saline, 50 mL of saturated NaHCO 3 Solution extraction 3 times, anhydrous MgSO 4 Dry, filter under reduced pressure to remove MgSO 4 Concentrate under reduced pressure, separate a...
Embodiment 3
[0073] Embodiment 3: Synthesis of deoxycholic acid-9-O-propionamide berberine: (A3)
[0074] (1) Synthesis of Berberine
[0075] Same as (1) in Example 1.
[0076] (2) Synthesis of 9-O-3-bromopropyl berberine
[0077] Same as (2) in Example 1.
[0078] (3) Synthesis of 9-O-3-aminopropyl berberine:
[0079] Same as (3) in Example 1.
[0080] (4) Synthesis of deoxycholic acid activated ester:
[0081] Add 196 mg (0.5 mmol) of deoxycholic acid to a 50 mL round bottom flask, add 10 mL of dichloromethane, add 115 mg of N-hydroxysuccinimide, and add 1-ethyl-3-(3-dimethylaminopropyl ) carbodiimide 766mg (4mmol) dichloromethane solution 10mL, follow the reaction by TLC. After the reaction was complete, 30 mL of chloroform was added to the reaction liquid, followed by 50 mL of saturated saline, 50 mL of saturated NaHCO 3 Solution extraction 3 times, anhydrous MgSO 4 Dry, filter under reduced pressure to remove MgSO 4 Concentrate under reduced pressure, separate and purify by s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com