Novel 7alpha-hydroxysteroid dehydrogenase knockout mutants and use thereof
A technology of hydroxysteroids and dehydrogenases, applied in the direction of enzymes, oxidoreductases, and the use of vectors to introduce foreign genetic materials, etc., can solve problems such as pollution and accumulation of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0031] 1. Recombinant microorganisms, wherein the enzymatic activity of 7α-hydroxysteroid dehydrogenase (7α-HSDH) is inhibited, and simultaneously expressively contain functionally different hydroxysteroid dehydrogenases (for example, 3α-, 3β-, 7β-, 11α -, 11β-, 12α-, 12β-, 17α-, 17β-, 20α- or 20β-HSDH, especially 3α-, 7β- or 12α-HSDH) enzymatic activity.
[0032] The 7α-HSDH inhibited here can show any desired cofactor dependence, for example, on NADH or NADPH (or NAD + or NADP + ) dependencies.
[0033] Optionally, this modified microorganism can also be additionally modified so that it not only expresses the desired HSDH activity, but also other proteins or enzymes that can optionally function in conjunction with HSDH, such as assisting cofactor regeneration Enzymes (as explained in more detail below). Examples of such enzymes are eg alcohol dehydrogenase (ADH) and formate dehydrogenase (FDH).
[0034] In particular, the present invention relates to recombinant microorg...
Embodiment 1
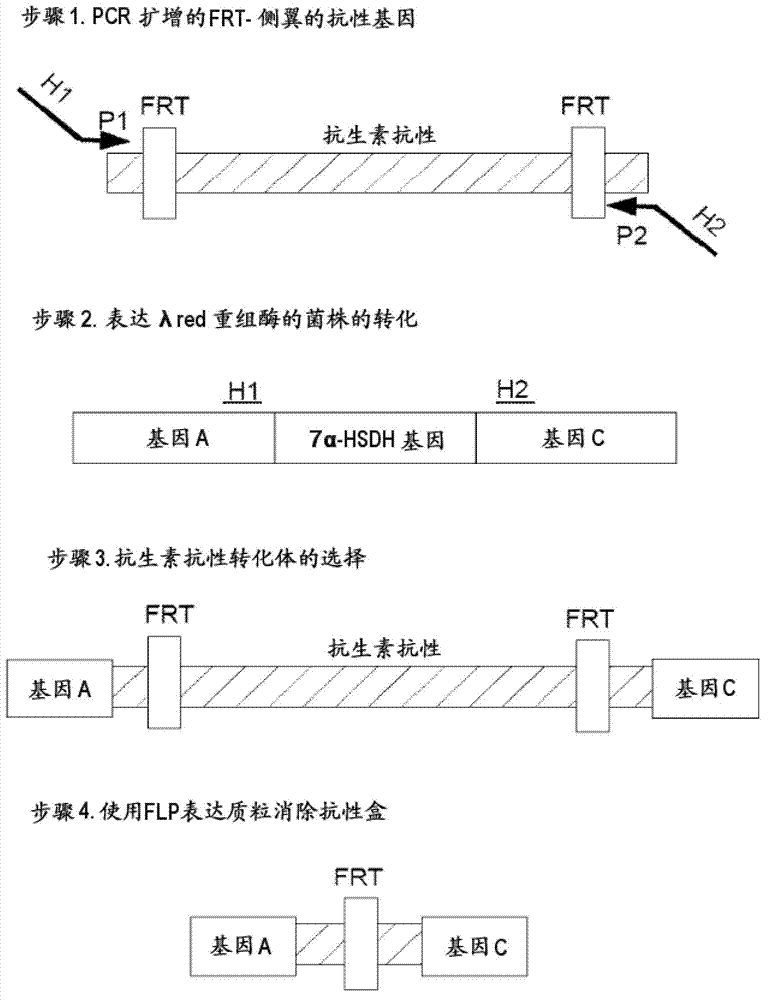
[0415] Example 1: Production of a 7α-HSDH deletion mutant of E. coli strain BL21(DE3) (E. coli BL21(DE3)Δ7α-HSDH) (type 1) (knockout by homologous recombination)
[0416] 1.1 Sequence information of 7α-HSDH from Escherichia coli BL21(DE3)
[0417] Amino acid sequence: (SEQ ID NO: 10)
[0418] Length: 255 amino acids
[0419] Type: protein
[0420] Source: Escherichia coli BL21 (DE3)
[0421] VFNSDNLRLDGKCAIITGAGAGIGKEIAITFATAGASVVVSDINADAANHVVDEIQQLGGQAFACRCDITSEQELSALADFAISKLGKVDILVNNAGGGGPKPFDMPMADFRRAYELNVFSFFHLSQLVAPEMEKNGGGVILTITSMAAENKNINMTSYASSKAAASHLVRNMAFDLGEKNIRVNGIAPGAILTDALKSVITPEIEQKMLQHTPIRRLGQPQDIANAALFLCSPAASWVSGQILTVSGGGVQELN
[0422] Nucleotide sequence (SEQ ID NO: 9)
[0423] Length: 768 base pairs
[0424] Type: nucleic acid
[0425] Source: Escherichia coli BL21 (DE3)
[0426] Accession number: NC_012971 Area: 1642470..1643237
[0427] gtgtttaatt ctgacaacct gagactcgac ggaaaatgcg ccatcatcac aggtgcgggt 60
[0428] gcaggtattg gtaaagaaat cgccattaca ttcg...
Embodiment 2
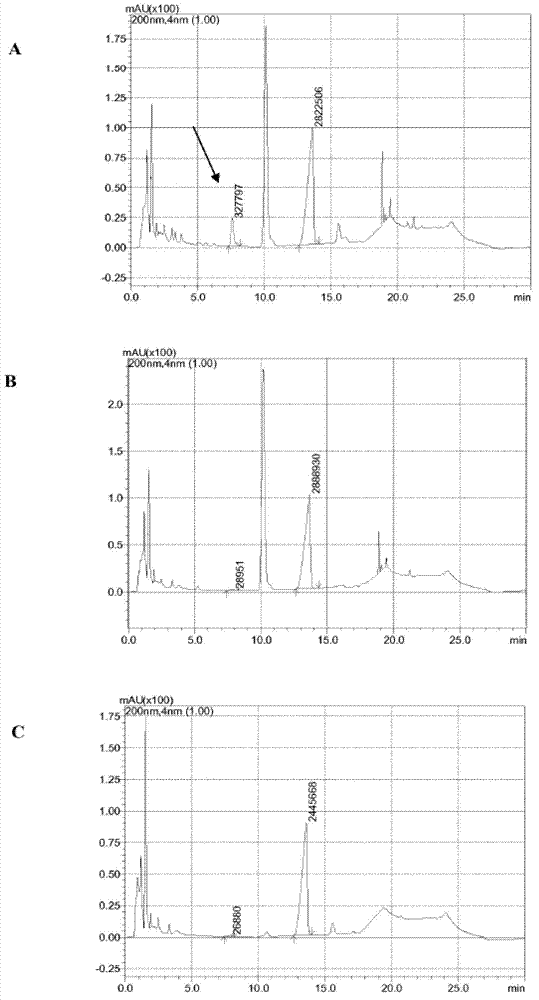
[0458] Example 2: Heterologous expression of 12α-HSDH using Escherichia coli BL21(DE3)Δ7α-HSDH (type 1)
[0459] The pET28a(+) vector, Novagen, Darmstadt, which contains the MCS under the control of the T7 promoter and the transcription start and the T7 terminator for the expression of 12α-HSDH. Expression is induced by isopropyl-β-D-thiogalactopyranoside (IPTG).
[0460] To this end, the sequence encoding 12α-HSDH (short form) was PCR amplified (see also Applicant's PCT / EP2009 / 002190). PCR products were obtained using genomic DNA of Clostridium group P strain 48–50 as template and the following primer pairs:
[0461] (5'-GGTATTCCATATGATCTTTGACGGAAAGGTCGC-3' (SEQ ID NO:5) and
[0462] 5'-CGGGATCCCTAGGGGCGCTGACCC-3') (SEQ ID NO: 6).
[0463] Nucleotide residues in italics are redundant. Residues in bold encode the cleavage site. The other residues are homologous sequences of 12α-HSDH.
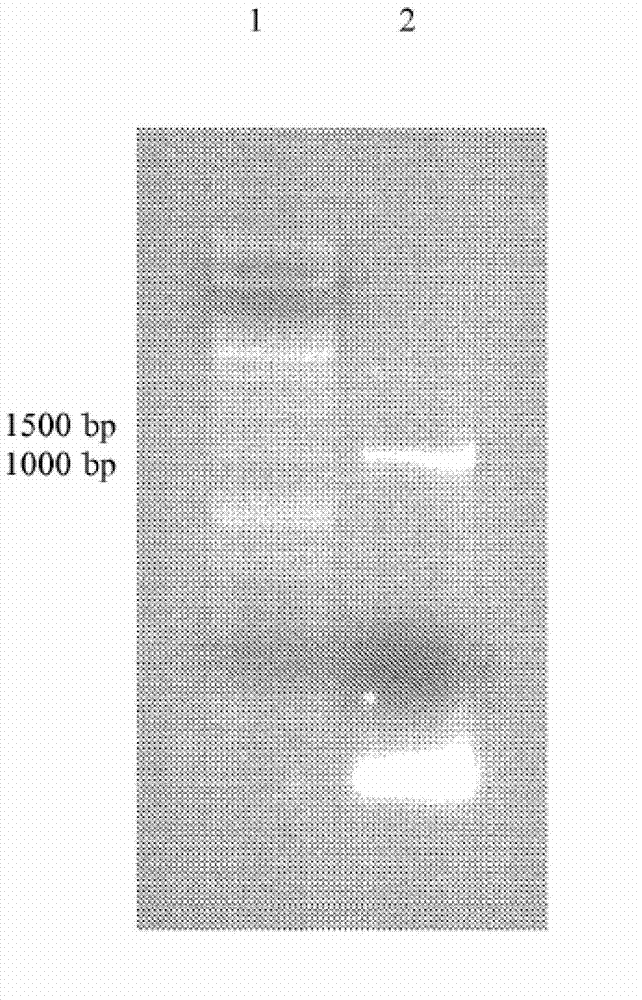
[0464] The PCR products were applied to an agarose gel, separated and excised from the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com