Application of taurochenodeoxycholic acid to protection of gastrointestinal tract function
A technology of taurine chenodeoxycholic acid and digestive tract, which is applied to the digestive system, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as difficulty in meeting modern technologies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
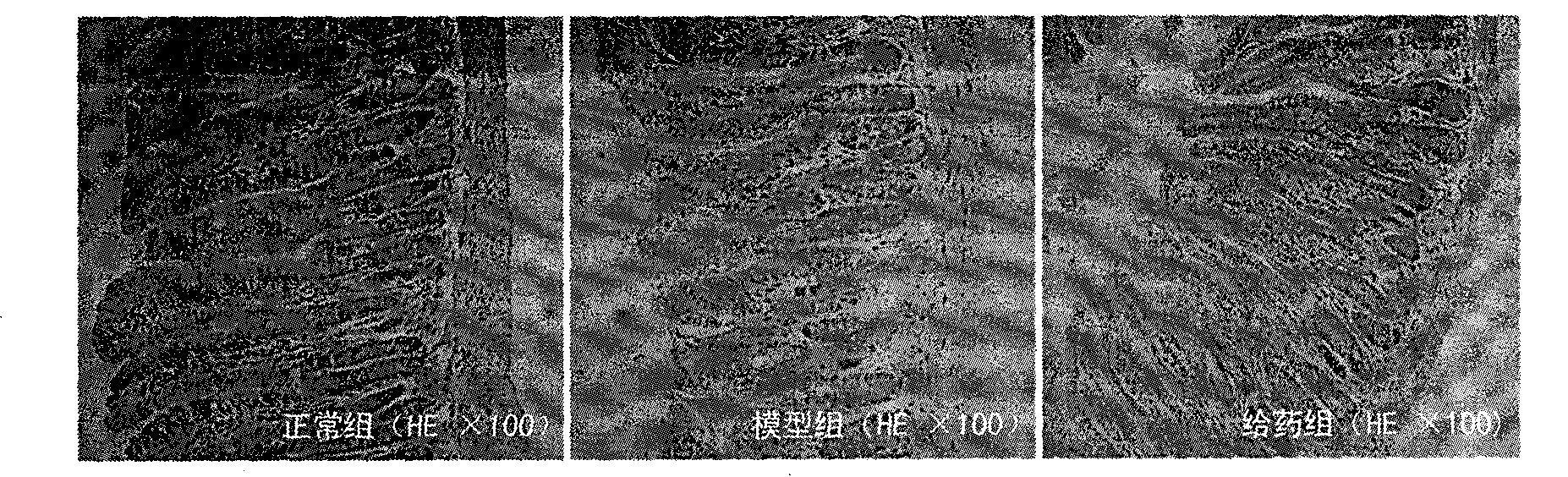
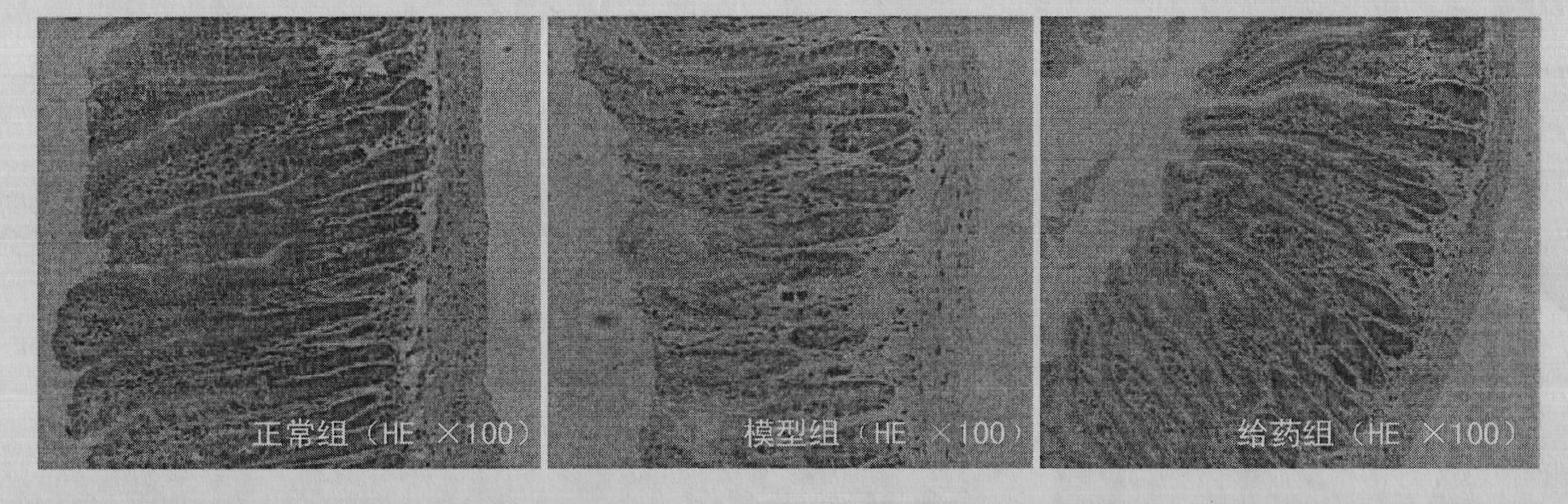
[0017] During the experiment, they were divided into normal group, model group and drug administration group, each with 10 mice. The normal group was only fed with distilled water; the model group was injected intraperitoneally with 80 mg / kg dexamethasone for 7 consecutive days to prepare the mouse model of gastrointestinal injury caused by glucocorticoids; the administration group was prepared in the model After completion of the administration, the dose of TCDCA in the administration group was 0.1 g / kg, and the route of administration was intragastric administration, once a day, for 7 consecutive days. HE staining of paraffin sections was used to evaluate the influence of TCDCA on the pathological changes of the digestive tract, the results are shown in the attached figure 1 And attached image 3 shown. In the model group, the small intestinal submucosa was loose and edema, with inflammatory cell infiltration, degeneration and necrosis of the small intestinal villi, and th...
Embodiment 2
[0019] During the experiment, they were divided into normal group, model group and drug administration group, each with 10 mice. The normal group was only fed with distilled water; the model group was injected intraperitoneally with 80 mg / kg dexamethasone for 7 consecutive days to prepare the mouse model of gastrointestinal injury caused by glucocorticoids; the administration group was prepared in the model After completion of the administration, the dose of TCDCA in the administration group was 0.1 g / kg, and the route of administration was intragastric administration, once a day, for 7 consecutive days. EDU was injected intraperitoneally into mice in each group, and the effect of TCDCA on the proliferation of digestive tract cells was detected by EdU DNA in vivo Kit. Observe under a fluorescence microscope at 200 times, excite and irradiate with ultraviolet light and green light respectively and take pictures. Three consecutive fields of view of small intestine tissue were s...
Embodiment 3
[0024] During the experiment, they were divided into normal group, model group and drug administration group, each with 10 mice. The normal group was only fed with distilled water; the model group was injected intraperitoneally with 80 mg / kg dexamethasone for 7 consecutive days to prepare the mouse model of gastrointestinal injury caused by glucocorticoids; the administration group was prepared in the model After completion of the administration, the dose of TCDCA in the administration group was 0.1 g / kg, and the route of administration was intragastric administration, once a day, for 7 consecutive days. The content of SlgA in the small intestine homogenate was detected by radioimmunoassay, and the results are shown in Table 2. Compared with the normal group, the medium-dose group can significantly promote the secretion of intestinal SIgA; compared with the model group, the low-, medium-, and high-dose groups can significantly or extremely significantly enhance the secretion o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com