7-ketolithocholic acid intermediate and preparation process and application thereof
A preparation process and a technology for an intermediate, which are applied to a 7-ketolithocholic acid intermediate and its preparation process and application field, can solve the problems of waste of resources, environment, separation difficulties, pollution and the like, achieve mild reaction conditions, simplify the reaction route, The effect of high safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
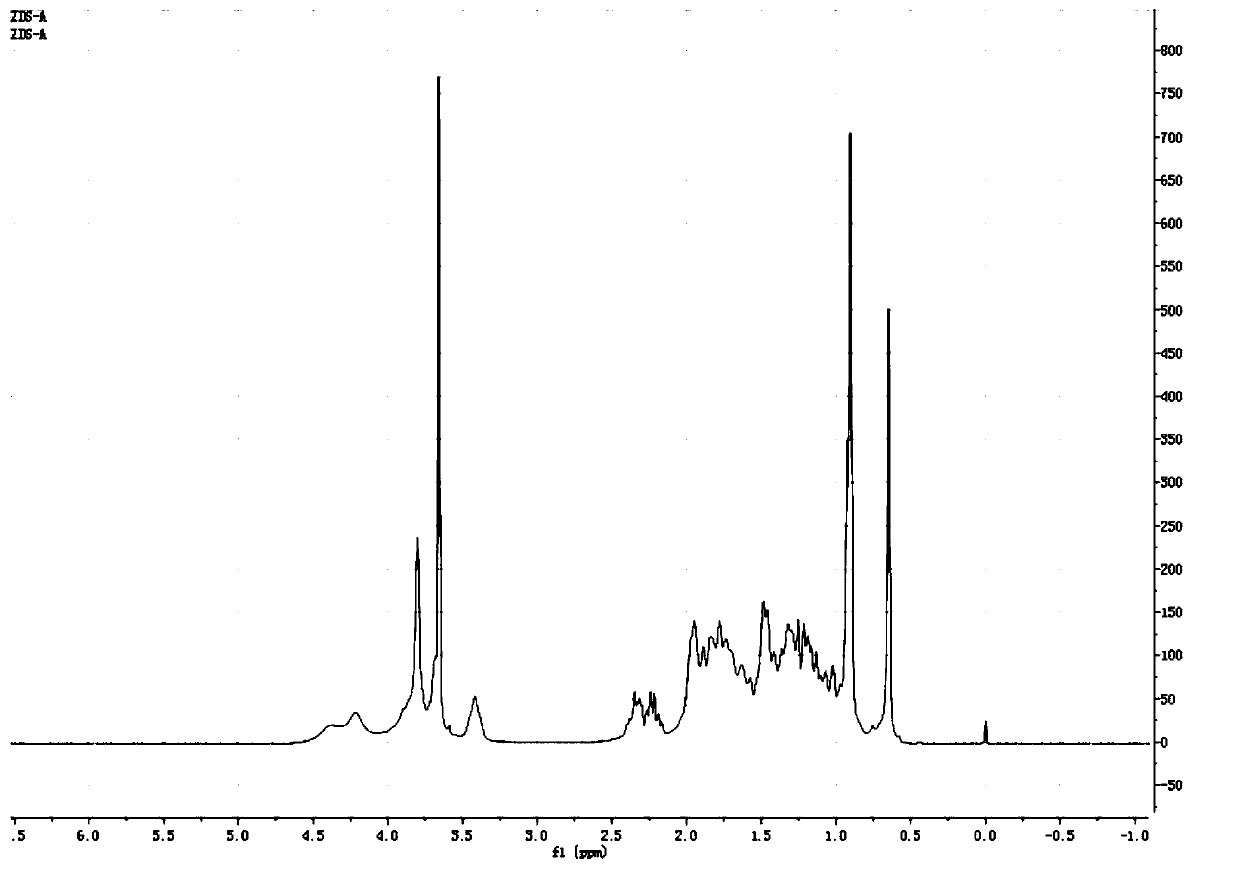
[0063] In this example, R 1 is methyl, R 2 is trimethylsilyl, R 3 For methanesulfonyl.
[0064] The preparation technology of 7-keto lithocholic acid intermediate comprises the following steps:
[0065] Step 1: Dissolve 5kg of hyocholic acid in 50kg of methanol, add 300g of sulfuric acid, heat up to reflux and stir for 3 hours, TLC monitors that the reaction is complete, stop the reaction, concentrate to remove the solvent, a large amount of solids are precipitated, filter to obtain intermediate 1, and vacuum-dry Obtain 5kg of white solid;
[0066] Step 2: Dissolve 2kg of intermediate 1 in 20kg of dichloromethane, add 2kg of trimethylchlorosilane and 1.2kg of triethylamine, heat up to reflux and stir for 2 hours, TLC monitors that the reaction is complete, stop the reaction, concentrate to remove the solvent, a large amount of Solids were precipitated, and intermediate 2 was obtained by filtration, and 2.1 kg of white solids were dried under vacuum at 50°C;
[0067] Step ...
Embodiment 2
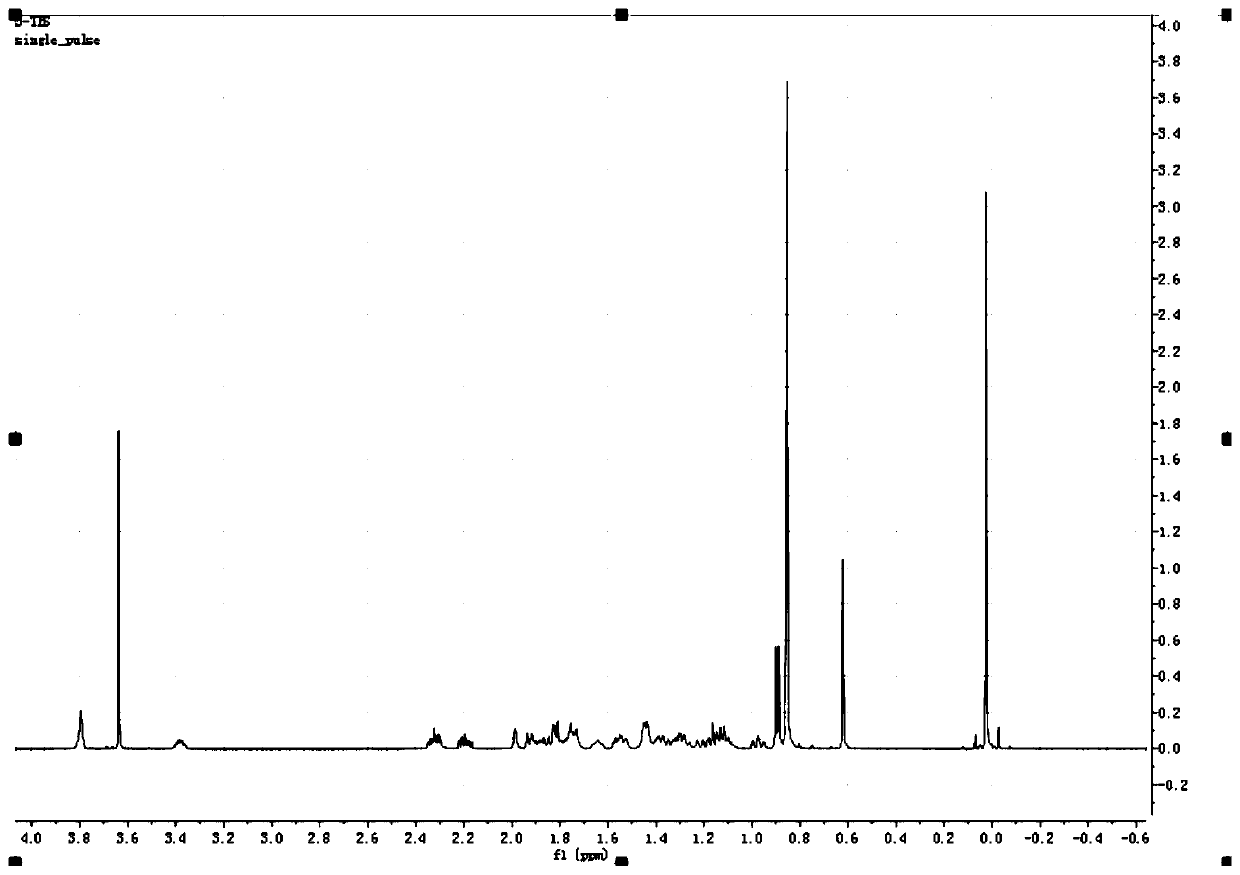
[0074] In this example, R 1 is methyl, R 2 is dimethyl tert-butylsilyl, R 3 For methanesulfonyl.
[0075] The preparation technology of 7-keto lithocholic acid intermediate comprises the following steps:
[0076] Step 1: Dissolve 5kg of hyocholic acid in 50kg of methanol, add 300g of sulfuric acid, heat up to reflux and stir for 3 hours, TLC monitors that the reaction is complete, stop the reaction, concentrate to remove the solvent, a large amount of solids are precipitated, filter to obtain intermediate 1, and vacuum-dry Obtain 5kg of white solid;
[0077] Step 2: Dissolve 2kg of intermediate 1 in 20kg of toluene, add 2kg of dimethyl tert-butylchlorosilane and 1.3kg of imidazole, heat up to reflux and stir for 2 hours, TLC monitors that the reaction is complete, stop the reaction, concentrate to remove the solvent, and a large amount of solid Precipitation, filtration to obtain intermediate 2, 2.1 kg of white solid dried in vacuum at 50°C;
[0078] Step 3: Dissolve 1kg ...
Embodiment 3
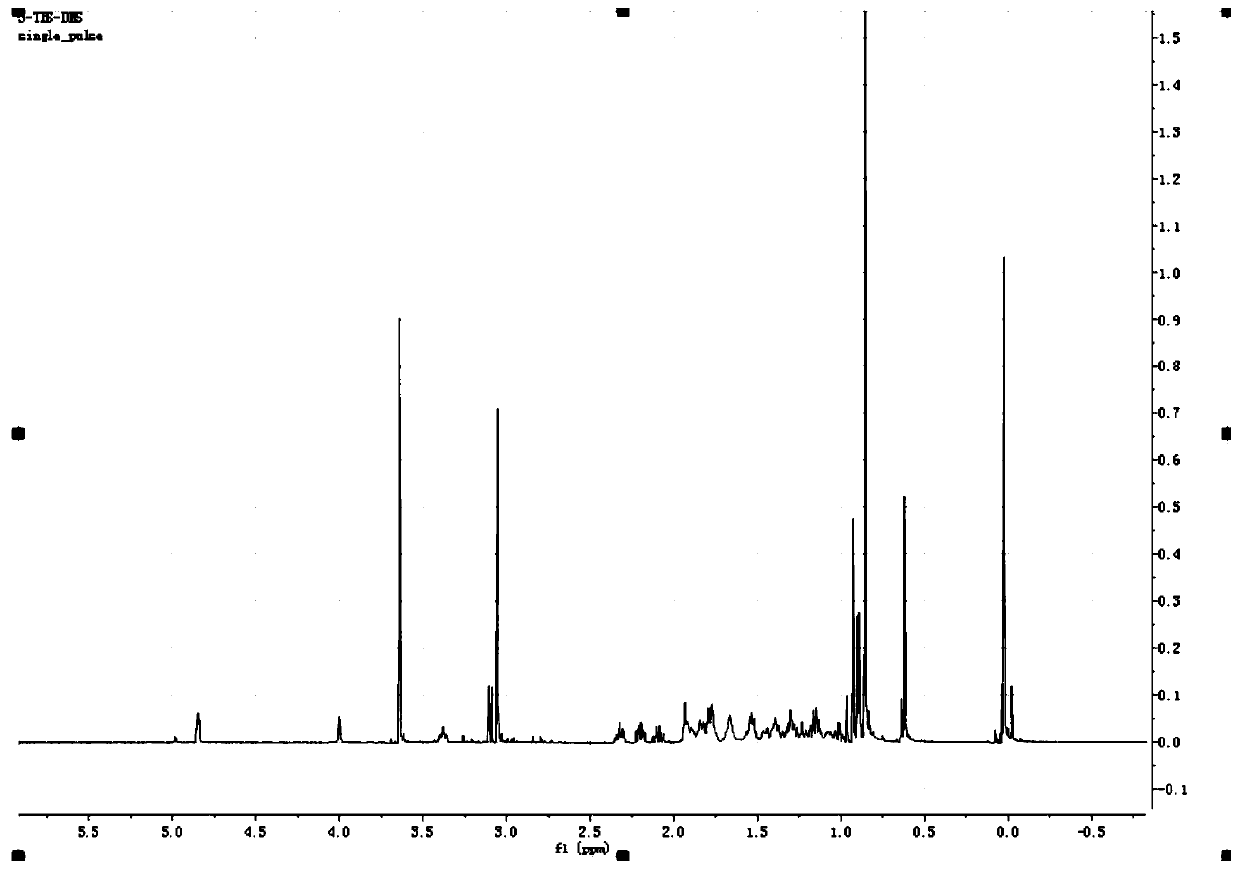
[0085] This embodiment is based on embodiment 1 or embodiment 2, and the difference with embodiment 1 or embodiment 2 is:
[0086] The second step: the preparation of intermediate 5:
[0087] Dissolve 10g of intermediate 4 in 200g of methyl isobutyl ketone, add 36.4g of lithium iodide, 16g of fully activated zinc powder and 10g of deionized water, reflux and stir for 72 hours, stop the reaction, TLC monitors that the reaction is complete, stop For the reaction, the reaction solution was dropped into 400 g of deionized water, a large amount of solids were precipitated, and intermediate 5 was obtained by filtration, and dried in vacuum at 50° C. to obtain 6.8 g of white solids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com