Preparation method of Huodan dropping pills
A technology for the preparation of dripping pills and Huodan, which is applied in the field of solid dispersion pharmaceutical preparations and Huodan dripping pills. It can solve the problems of drug safety risks and slow addition, and achieve shortening of heating time, halving of heating time, and loss of volatilization Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
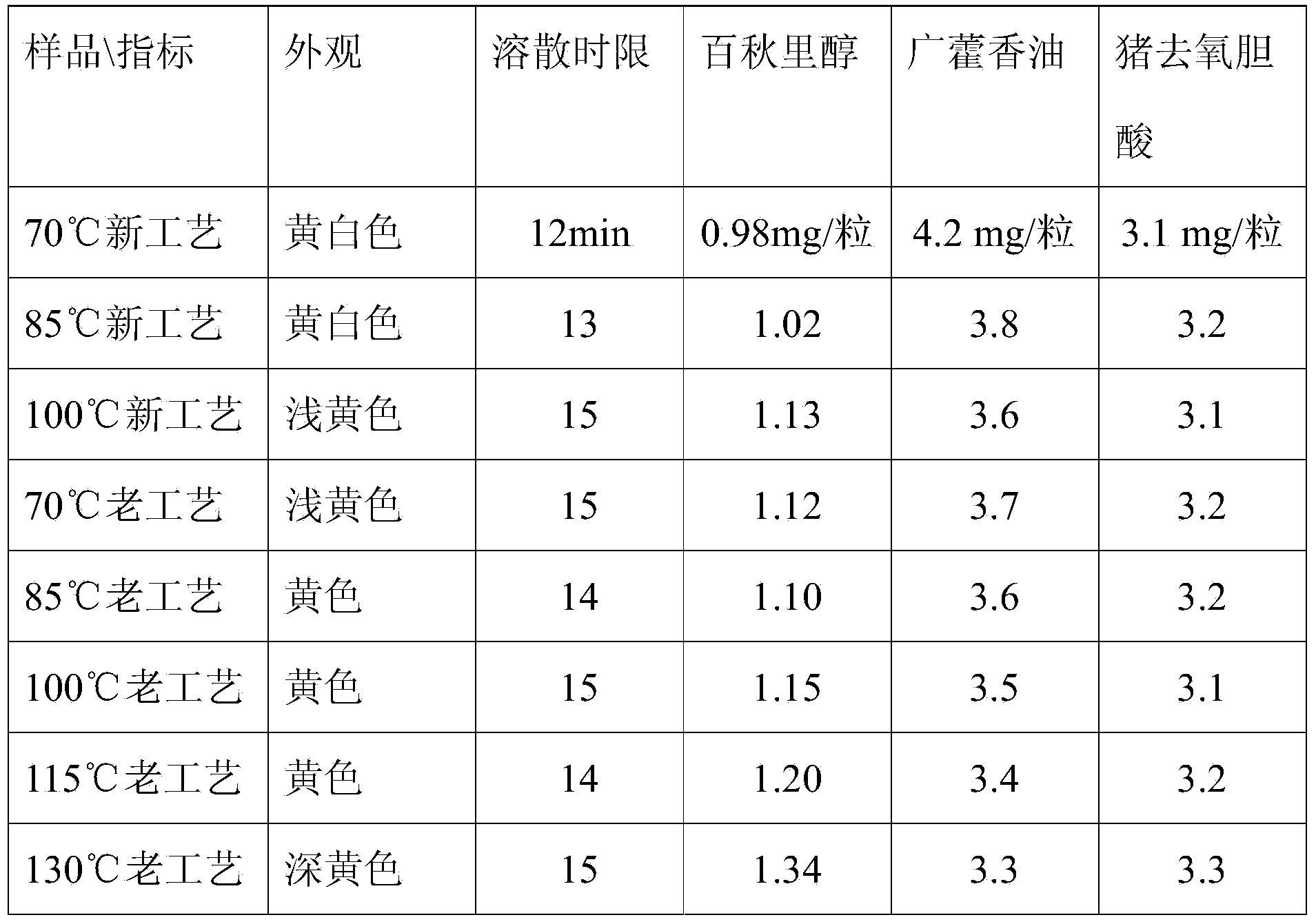
[0032] Determination of hyodeoxycholic acid in Huodan dripping pills in embodiment 1
[0033] Measured according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2010 Edition).
[0034] Chromatographic conditions and system suitability test used octadecylsilane bonded silica gel as filler; acetonitrile-0.1% glacial acetic acid (50:50) as mobile phase; evaporative light scattering detector. The number of theoretical plates calculated based on hyodeoxycholic acid peak should not be less than 3500.
[0035] Preparation of Reference Substance Solution Accurately weigh an appropriate amount of hyodeoxycholic acid reference substance that has been dried under reduced pressure with phosphorus pentoxide for 24 hours, weigh it accurately, add methanol to make a solution containing 0.4mg per 1ml, and obtain it.
[0036] The preparation of the test solution takes an appropriate amount of this product under the weight difference item, grinds finely, takes ...
Embodiment 2
[0038] Determination of patchouli oil in embodiment 2 Huodan dripping pills
[0039] According to gas chromatography (Chinese Pharmacopoeia Appendix VIE) determination.
[0040] Chromatographic conditions and system suitability test A capillary column with 5% phenylmethyl polysiloxane as the stationary phase (column length is 30m, inner diameter is 0.32mm, film thickness is 0.25μm); column temperature is programmed temperature: initial temperature 120°C, hold for 5 minutes, raise the temperature to 170°C at a rate of 10°C per minute, hold for 4 minutes; the detector temperature is 280°C, the inlet temperature is 280°C; split injection, the split ratio is 10:1. The number of theoretical plates should not be less than 5000 based on the peak of patchouli oil.
[0041] Preparation of Reference Substance Solution Use patchouli oil raw material as reference substance, take an appropriate amount, accurately weigh it, add ethyl acetate to make a solution containing 2 mg per 1 ml, and...
Embodiment 3
[0044] Buchuliol content determination in embodiment 3 Huodan dripping pills
[0045] Determination according to gas chromatography (Appendix VIE).
[0046] Chromatographic conditions and system suitability test A capillary column with 5% phenylmethylpolysiloxane as the stationary phase (column length is 30m, inner diameter is 0.32mm, film thickness is 0.25μm); column temperature is programmed temperature: initial temperature 120°C, hold for 5 minutes, raise the temperature to 170°C at a rate of 10°C per minute, hold for 4 minutes; the detector temperature is 280°C, the inlet temperature is 280°C; split injection, the split ratio is 10:1. The number of theoretical plates should not be less than 5000 based on the calculation of the alcohol peak of potion.
[0047] Preparation of Reference Substance Solution: Take an appropriate amount of the reference substance of potcine alcohol, accurately weigh it, add ethyl acetate to make a solution containing 0.6mg per 1ml, and obtain it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com