Method for synthesizing ursodesoxycholic acid by using blanking material after extraction of bilirubin from pig gall
A technology of ursodeoxycholic acid and bilirubin, which is applied in the field of synthesis of ursodeoxycholic acid, can solve the problems of production capacity accumulation, environmental pollution, less application of hyocholic acid, etc., and achieve the effect of improving the conversion yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
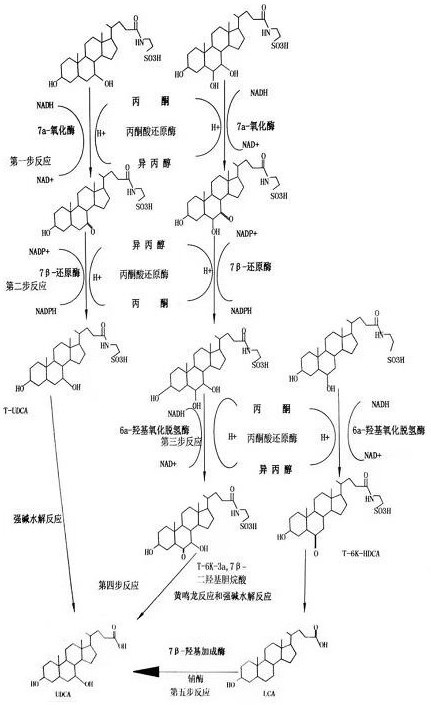
[0021] A method for synthesizing ursodeoxycholic acid from pig gall to extract bilirubin, the process steps are as follows:
[0022] 1. Take 1000g of pig bile after extracting bilirubin, add 0.1g of 7α-cholanate dehydrogenase to 1000g of pig bile, and add 0.01g of coenzyme NAD + , add 0.2g acetone reductase, use acetone reductase to reduce acetone to isopropanol and accept H source, the temperature during the reaction is controlled at 25°C, T-CDCA is oxidized to T-7K-LCA, T-HCA is oxidized to T-7K-HCA, through HPLC and TLC inspection, T-CDCA and T-HCA residues are less than 1%, the reaction is terminated, the total conversion rate is greater than 95%, and it is ready for use.
[0023] 2. Add 0.1g 7β-cholanate hydrogenase and 0.01g coenzyme NADP to the spare oxidation product in step 1 + , the temperature is controlled at 35°C, 10g of isopropanol is added, the isopropanol is oxidized to acetone to provide H source, and the acetone is recovered by vacuuming under stirring, so t...
Embodiment 2
[0030] A method for synthesizing ursodeoxycholic acid from pig gall to extract bilirubin, the process steps are as follows:
[0031] 1. Take 1000g of pig bile after extracting bilirubin, add 0.2g of 7α-cholanate dehydrogenase to 1000g of pig bile, and add 0.02g of coenzyme NAD + , add 0.3g acetone reductase, use acetone reductase to reduce acetone to isopropanol and accept H source, the temperature during the reaction is controlled at 28°C, T-CDCA is oxidized to T-7K-LCA, T-HCA is oxidized to T-7K-HCA, through HPLC and TLC inspection, T-CDCA and T-HCA residues are less than 1%, the reaction is terminated, the total conversion rate is greater than 95%, and it is ready for use.
[0032] 2. Add 0.2g 7β-cholanate hydrogenase and 0.02g coenzyme NADP to the spare oxidation product in step 1 + , the temperature is controlled at 37°C, 15g of isopropanol is added, the isopropanol is oxidized to acetone to provide H source, and the acetone is recovered by vacuuming under stirring, so t...
Embodiment 3
[0039] A method for synthesizing ursodeoxycholic acid from pig gall to extract bilirubin, the process steps are as follows:
[0040] 1. Take 1000g of pig bile after extracting bilirubin, add 0.3g of 7α-cholanate dehydrogenase to 1000g of pig bile, and add 0.03g of coenzyme NAD + , add 0.4g acetone reductase, use acetone reductase to reduce acetone to isopropanol and accept H source, the temperature during the reaction is controlled at 30°C, T-CDCA is oxidized to T-7K-LCA, T-HCA is oxidized to T-7K-HCA, through HPLC and TLC inspection, T-CDCA and T-HCA residues are less than 1%, the reaction is terminated, the total conversion rate is greater than 95%, and it is ready for use.
[0041] 2. Add 0.3g of 7β-cholanate hydrogenase and 0.03g of coenzyme NADP to the standby oxidation product in step 1 + , the temperature is controlled at 38°C, and 20g of isopropanol is added to oxidize isopropanol to acetone to provide H source, and the acetone is recovered by vacuuming under stirring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com