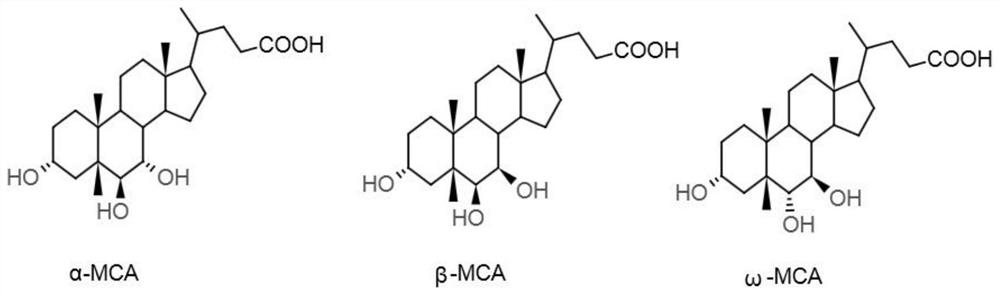
Preparation method of alpha-murine cholic acid
A technology for murine cholic acid and cholanoic acid, applied in the field of organic chemical synthesis, can solve the problems of high process cost of murine acid, unsuitable for mass production, difficult process and the like, and achieves a wide range of raw material sources, few impurities and simple reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
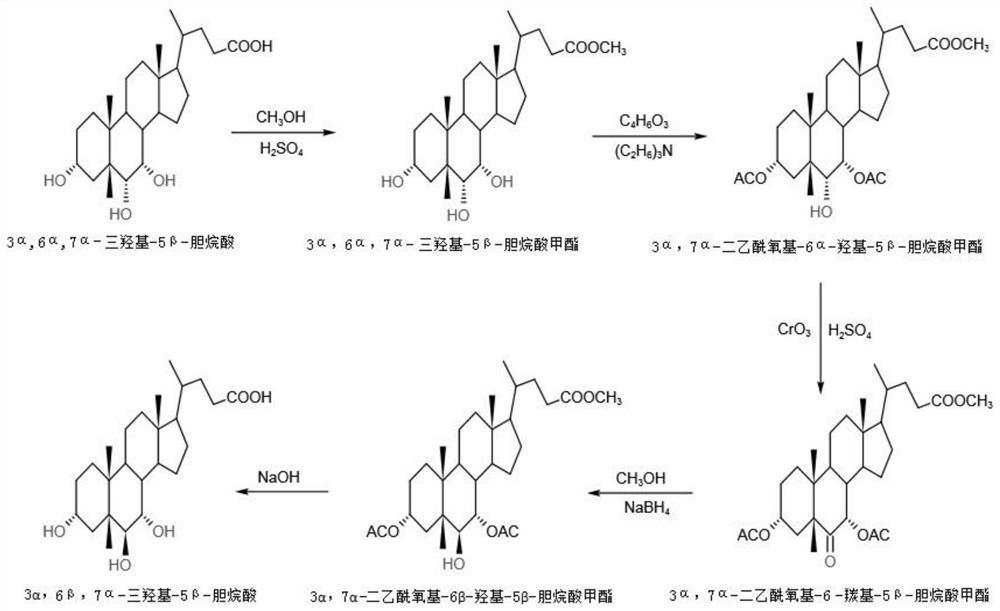
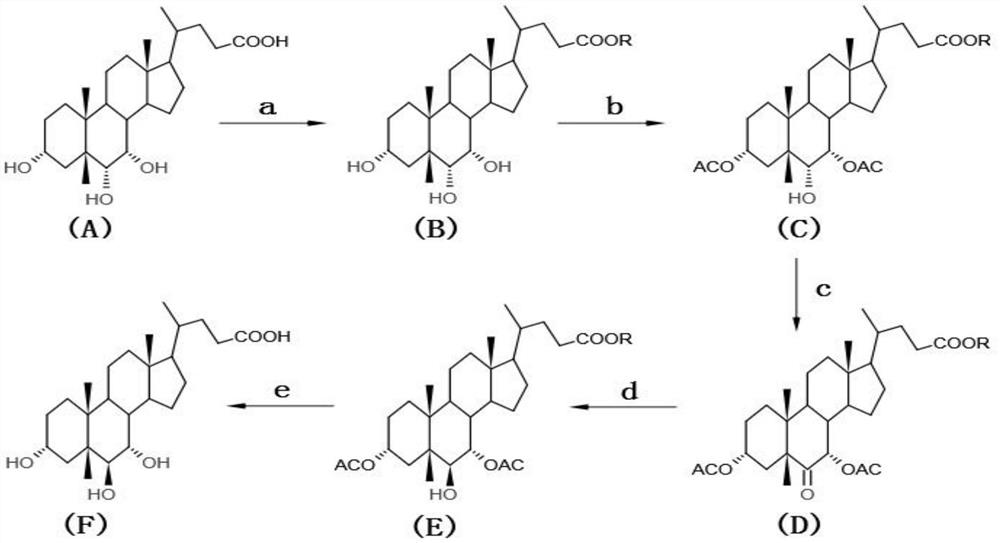
[0051] The preparation method of murichic acid of the present embodiment, refer to figure 2 Shown synthetic route map, comprises the following steps:
[0052] a. Preparation of 3α, 6α, 7α trihydroxy-5β-cholanoic acid methyl ester
[0053] In a 500ml three-neck flask, add 50g of 3α, 6α, 7α trihydroxy-5β-cholic acid (hyocholic acid), 200ml of methanol and stir to dissolve, add 0.25ml of concentrated sulfuric acid, heat and reflux for 2 hours, and take a sample to measure the residual hyocholic acid. 0.732%. Add 50ml of sodium bicarbonate solution with a concentration of 1%, stir evenly, distill in vacuum, remove methanol, obtain a large amount of white crystals, filter, wash the filter cake with 200ml of water, dry in a hot air circulation oven at 75°C for 8 hours, and obtain 3α, 6α, 7α Hydroxy-5β-cholanic acid methyl ester 49.8g, HPLC detection purity 98.365%.
[0054] b. Preparation of 3α, 7α-diacetoxy-6α-hydroxy-5β-cholanoic acid methyl ester
[0055]Add 49.8g of 3α, 6α,...
Embodiment 2
[0065] a. Preparation of 3α, 6α, 7α trihydroxy-5β-cholanoic acid methyl ester
[0066] In a 500ml three-necked flask, add 50g of 3α, 6α, 7α trihydroxy-5β-cholic acid (hyocholic acid), 300ml of methanol and stir to dissolve, add 1.0ml of concentrated hydrochloric acid, heat and reflux for 3 hours, and take a sample to measure the residual hyocholic acid. 0.617%. Add 25ml of sodium bicarbonate solution with a concentration of 1%, stir evenly, and vacuum distill to remove methanol to obtain a large amount of white crystals, filter, wash the filter cake with 300ml of water, and dry in a hot air circulation oven at 80°C for 6h to obtain 3α, 6α, 7α Hydroxy-5β-cholanic acid methyl ester 49.6g, HPLC detection purity 99.547%.
[0067] b. Preparation of 3α, 7α-diacetoxy-6α-hydroxy-5β-cholanoic acid methyl ester
[0068] Add 49.6g of 3α, 6α, 7α trihydroxy-5β-cholanoic acid methyl ester and 400ml of dichloromethane into a dry 1000ml three-necked flask, add 0.75g of 4-dimethylaminopyridi...
Embodiment 3
[0078] a. Preparation of 3α, 6α, 7α trihydroxy-5β-cholanoic acid ethyl ester
[0079] In a 500ml three-necked flask, add 50g of 3α, 6α, 7α trihydroxy-5β-cholic acid (hyocholic acid), 350ml of ethanol and stir to dissolve, add 0.5ml of concentrated sulfuric acid, heat up and reflux for 2.5h, and take a sample to measure the residual hyocholic acid is 0.805%. Add 80ml of sodium bicarbonate solution with a concentration of 1%, stir evenly, distill in vacuum, remove ethanol, obtain a large amount of white crystals, filter, wash the filter cake with 200ml of water, dry in a hot air circulation oven at 80°C for 7 hours, and obtain 3α, 6α, 7α Hydroxy-5β-cholanoic acid ethyl ester 49.5g, HPLC detection purity 99.679%.
[0080] b. Preparation of ethyl 3α, 7α-diacetoxy-6α-hydroxy-5β-cholanate
[0081] In a dry 1000ml three-necked flask, add 49.5g of 3α, 6α, 7α trihydroxy-5β-cholanoic acid ethyl ester from the previous step, 300ml of dichloromethane, add 0.25g of 4-dimethylaminopyridin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com