Adsorbing material of hepatitis B antigen protein and preparation method of material
An antigenic protein, hepatitis B technology, applied in the field of medical biomaterials, can solve the problems of cumbersome and complicated antibody production, many steps in the preparation process, and dangerous biological safety, so as to reduce the risk of immune rejection, simple and safe synthesis, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Expression and purification of human recombinant receptor protein
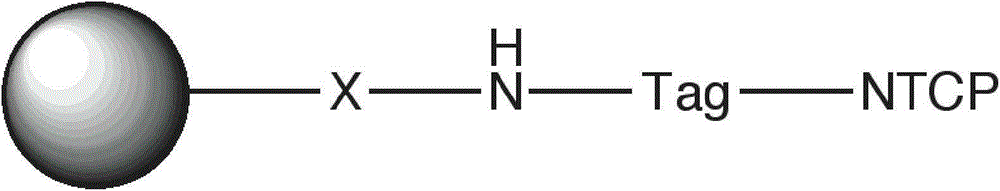
[0034] a) download the data NM_003049 from GenBank, and obtain the coding sequence of the NTCP polypeptide therefrom;
[0035] b) genetically improve the obtained coding sequence to obtain the corresponding prokaryotic expression sequence, the sequence is as follows:
[0036] 5’atggaagcacacaacgcgtctgcccctttcaacttcactctgccaccgaactttggcaaacgtccaacggacctggcactgtctgtaatcctggtattcatgctgttcttcattatgctgtctctgggttgcactatggagttctccaaaatcaaagcacatctgtggaaaccgaagggtctggcgattgcgctggtagcacagtatggcatcatgccactgactgctttcgttctgggtaaagtattccgtctgaaaaacattgaagcactggcgatcctggtatgtggttgctctccgggcggtaacctgtccaacgtgttctctctggccatgaaaggcgacatgaatctgagcatcgttatgactacttgcagcaccttttgcgctctgggcatgatgccgctgctgctgtacatctattctcgcggcatctacgatggtgatctgaaagacaaggttccgtataaaggtatcgtgatctccctggttctggtgctgatcccgtgcaccatcggtatcgttctgaagagcaaacgtccgcaatacatgcgctacgttattaaaggcggcatgatcattatcctgctgtgctctgtggcggtcaccgtcctgtctgccatcaac...
Embodiment 2
[0054] Example 2: Preparation of Adsorbent Material and Immobilization of Receptor Protein
[0055] Epoxy-active agarose gel (Sepharose CL-4B) was prepared using ethylene glycol diglycidyl ether as a coupling agent.
[0056] Add 600ml of agarose gel and 400ml of 2mol / L NaOH aqueous solution into a 2L reactor, mix well, add 400ml of ethylene glycol bisglycidyl ether, place in a constant temperature shaker, and react at 37°C for 1 hour. After the reaction was completed, the gel was filtered and rinsed with a large amount of distilled water until pH=7. Store the activated medium at 4°C for later use. The number of epoxy groups in the gel was detected by the sodium thiosulfate method, and it was measured that there were at least 40 μmol of active groups per milliliter.
[0057] Add 200ml of epoxy-activated agarose gel (Sepharose CL-4B) synthesized in this example, 600ml of 0.3mol / L boric acid buffer solution into a 2L reactor, control the pH value within the range of 8.0 to 10.0...
Embodiment 3
[0058] Example 3: Using commercialized hepatitis B antigen as the adsorption object to detect the performance of the adsorption material
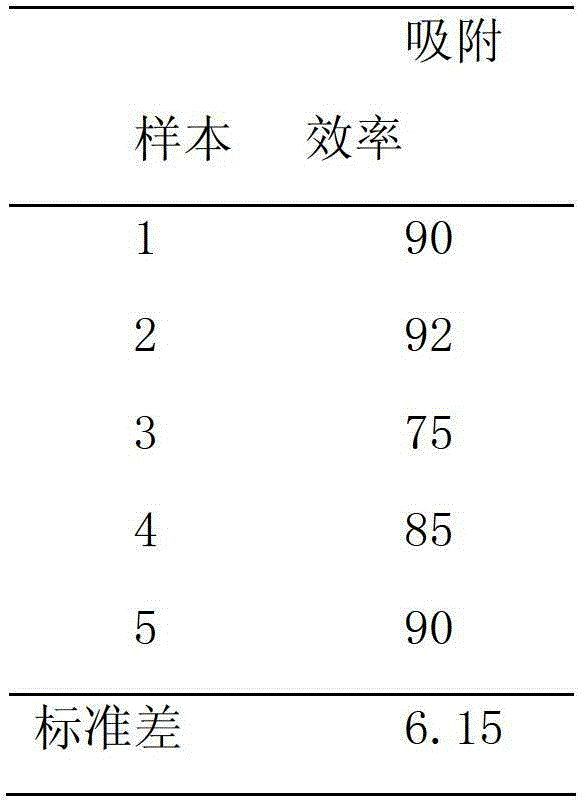
[0059] Inject 5 ml of the adsorption material obtained in Example 2 into a 500 ml Erlenmeyer flask, and add 100 ml of the PBS solution of hepatitis B antigen. React in a constant temperature shaker, shake at a low speed of 37°C for 90 minutes, use the ultraviolet method to detect the concentration of hepatitis B toxic protein, and take the ratio to determine the adsorption efficiency. The results are shown in Table 3-1
[0060] Adsorption efficiency of adsorption material to purified antigen under table 3-1 shaking table condition
[0061]
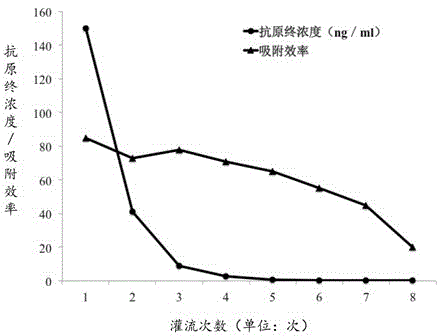
[0062] Inject 5ml of the adsorbent material obtained in Example 2 onto the filter membrane of the chromatography column, and communicate with it with a flexible pipe. Recover the filtered hepatitis B antigen solution with a clean triangular flask. The filtered solution was re-injected into the chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com