Hepatitis c virus adsorbent as well as preparation method and application thereof
A technology of hepatitis C virus and adsorbent, which is applied in the field of medical biomaterials to achieve the effect of good blood compatibility, strong specificity, and short safe process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 in situ PCR method prepares hepatitis C virus adsorbent
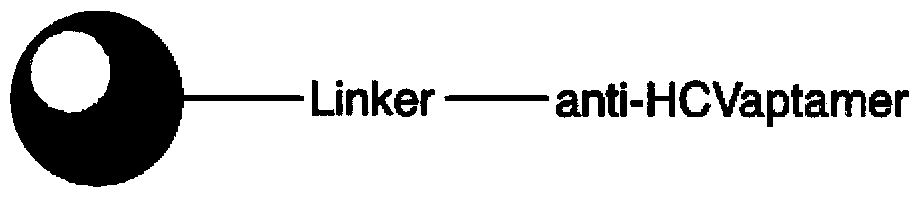
[0037] (1) Synthesize a single-stranded deoxyribonucleic acid ligand that binds to the envelope protein of hepatitis C virus. The sequence is as follows: 5'-gggatccccgCGCCCTAGGATACTGCGTAACTGGGAGTCGCTTCCTCGATCTAATAAGGAG TAAGCCGAGCCTGACAAGGGATATCACTCAGCATAATCTTAAGGCGaacgcgtctg-3'.
[0038] (2) Design primers to amplify the single-stranded deoxyribonucleic acid ligand binding to hepatitis C virus envelope protein, primer A: 5'-gggatccccgCGCCCTA-3', primer B: 5'-cagacgcgttCGCCTTA-3'.
[0039] (3) Entrust General Electric Company to connect primer A to high-flow agarose microsphere 6FF by chemical cross-linking method, use ligand as template, support carrier as one end primer, and primer B as opposite primer for in situ PCR reaction , so that the primer A immobilized on the carrier grows to the sequence described in step (1).
[0040] The system composition of the in situ PCR reaction is basically the sa...
Embodiment 2
[0043] Embodiment 2 Asymmetric PCR method prepares hepatitis C virus adsorbent
[0044] (1) and (2) are the same as embodiment 1;
[0045] (3) Convert the single-stranded deoxyribonucleic acid ligand bound to the hepatitis C virus envelope protein into double-stranded DNA by PCR reaction, and insert the double-stranded DNA into the cloning vector pUC19 for preservation; use the cloning vector or double-stranded DNA as The template was subjected to an asymmetric PCR reaction to obtain the target single-stranded DNA; the asymmetric PCR reaction conditions were as follows: the content of primer A was more than 100 times that of primer B; PCR reaction system: TaKaRa Ex Taq (5U / μL) 0.5μL, 10× Ex Taq Buffer 5 μL, dNTP Mixture (2.5 mM each) 4 μL, template DNA 1 μL, primer A (25 μmol / L) 2 μL, primer B (0.25 μmol / L) 2 μL, ddH 2 O 35.5 μL. PCR reaction program: 94°C for 3min; 94°C for 30s, 62°C for 90s, 32 cycles; 72°C for 20min to obtain single-stranded DNA.
[0046] (4) After purif...
Embodiment 3
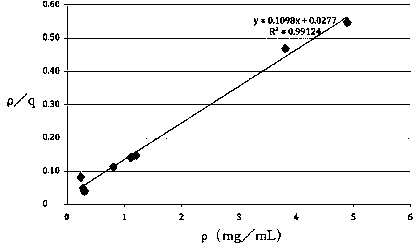
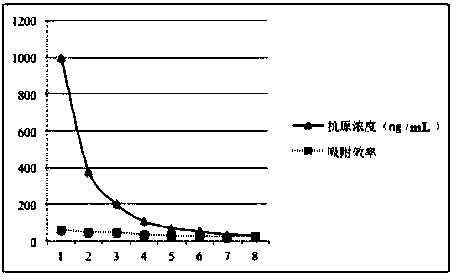
[0051] Example 3 The Hepatitis C Virus Adsorbent Measures the Maximum Adsorption Capacity of Hepatitis C Antigen E2
[0052] (1) Determination of standard curve of hepatitis C E2 concentration
[0053] Accurately weigh 25mg of HCV E2 standard substance, and prepare 25mL of 1.0mg / mL HCV E2 original solution with 0.01M PBS buffer solution with pH=7.4. Diluted with PBS to 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0 mg / mL of HCV E2 solution, 4 mL each. Using PBS as a blank control, the absorbance at 280 nm of each solution was detected. Draw the standard curve with the mass concentration as the abscissa and the absorbance as the ordinate, and the relationship between the two is y=2.31x through linear fitting; R 2 =0.9973.
[0054] (2) Hepatitis C E2 static adsorption
[0055] Use PBS (0.01M pH7.4) buffer to prepare HCV E2 solutions with a certain mass concentration gradient, the mass concentrations are 0.5, 0.81, 1.0, 1.5, 1.87, 2.0, 4.62, 5.78 mg / mL, and take 10 mL of HCV E2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com