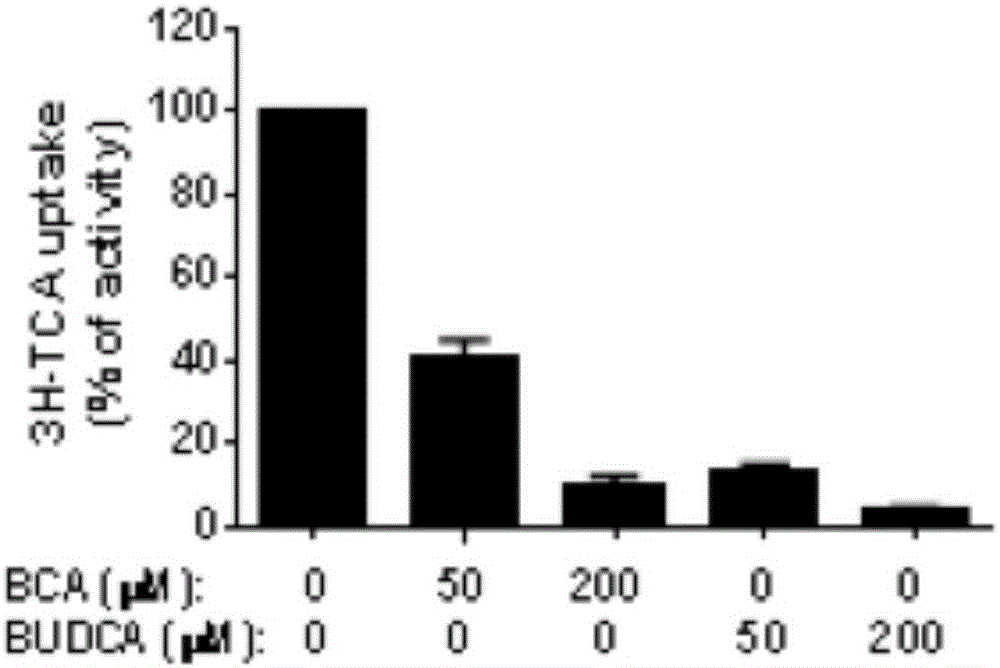
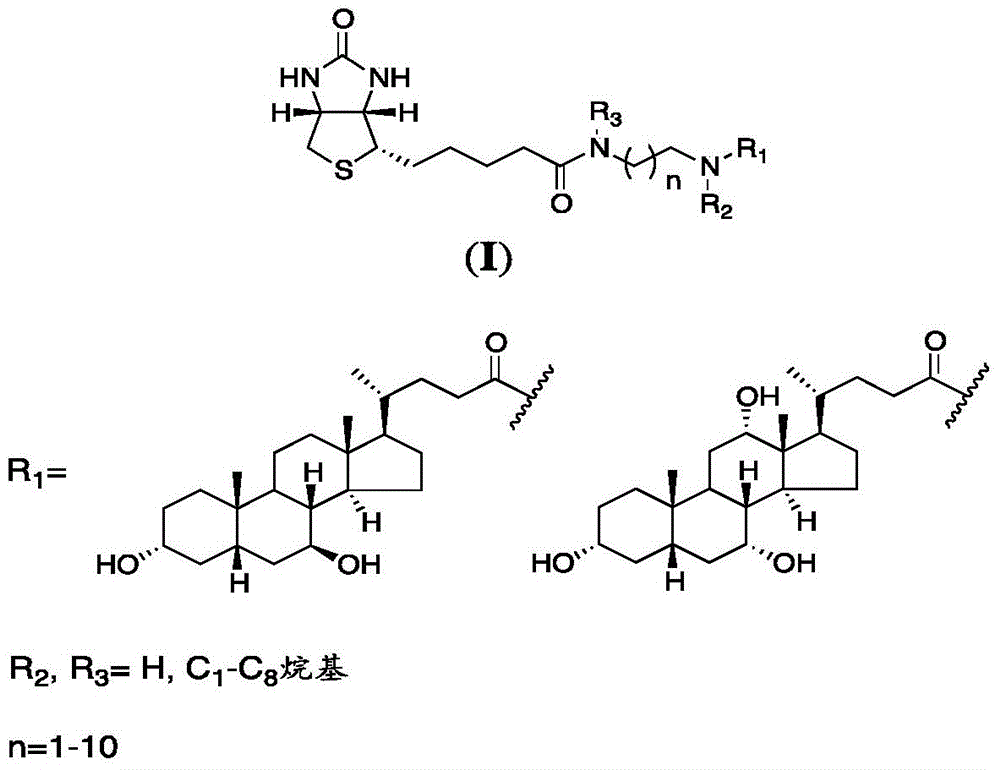
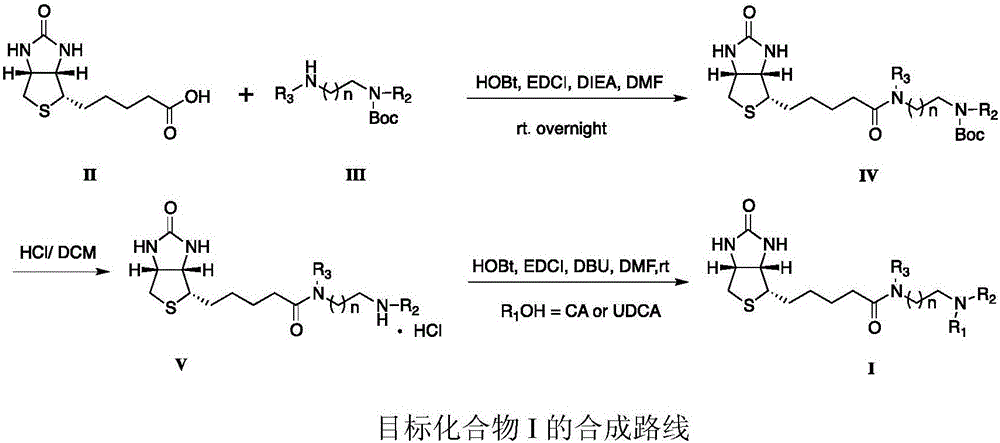
Sodium ion taurocholic acid co-transporter peptide inhibitor
A technology of polypeptide inhibitors and ion cattle, applied in the field of sodium ion taurocholic acid co-transport polypeptide inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] "Example 1" Synthesis of N-Boc-N-methylethylenediamine-biotin (IV)
[0025] Biotin (II, 11.68g, 47.82mM), 1.5eq HOBt (9.69g), 2.0eq EDCI (18.34g) were added to 25mL of anhydrous dry DMF, and stirred for 2h. Then 2.0eq DIEA (15.78mL) and N-Boc-N-methylethylenediamine (III, 10.0g, 57.38mM) were added and stirred overnight. Add 500ml of water to quench. The reaction solution was extracted with DCM (3×150 mL), washed with 1N HCl (3×100 mL), 1N NaOH (3×100 mL) and saturated brine (2×100 mL). Anhydrous Mg for organic layer 2 SO 4 Dry, filter and spin dry to obtain white solid IV (14.52g), yield: 75.82%.
Embodiment 2
[0026] "Example 2" (V) synthesis of N-methylethylenediamine-biotin
[0027] Dissolve IV (10.01g, 25mM) in dichloromethane (60mL), pass dry hydrochloric acid gas at room temperature for 2.5h, and continue stirring for 1.5h. The reaction solution was spin-dried and vacuum-dried to obtain white solid V (8.34 g), yield: 99.1%.
Embodiment 3
[0028] "Example 3" Synthesis of CA-N-methylethylenediamine-biotin (I-1)
[0029] Add cholic acid (2.04g, 5mM), 1.5eq HOBt (1.08g), 2.0eq EDCI (1.98g) into 10mL of anhydrous dry DMF, and stir for 2h. Then 3.6eq DBU (2.79g) and V (1.52g, 4.51mM) were added and stirred overnight.
[0030] Add 500ml of water to quench. The reaction solution was extracted with DCM (3×30 mL), washed with 1N HCl (3×30 mL), 1N NaOH (3×30 mL), and saturated brine (2×40 mL), respectively. Anhydrous Mg for organic layer 2 SO 4 Dry, filter, and spin dry to obtain a yellow oily liquid, which is purified by a silica gel column to obtain I-1 as a white solid (1.76 g), yield: 51.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com