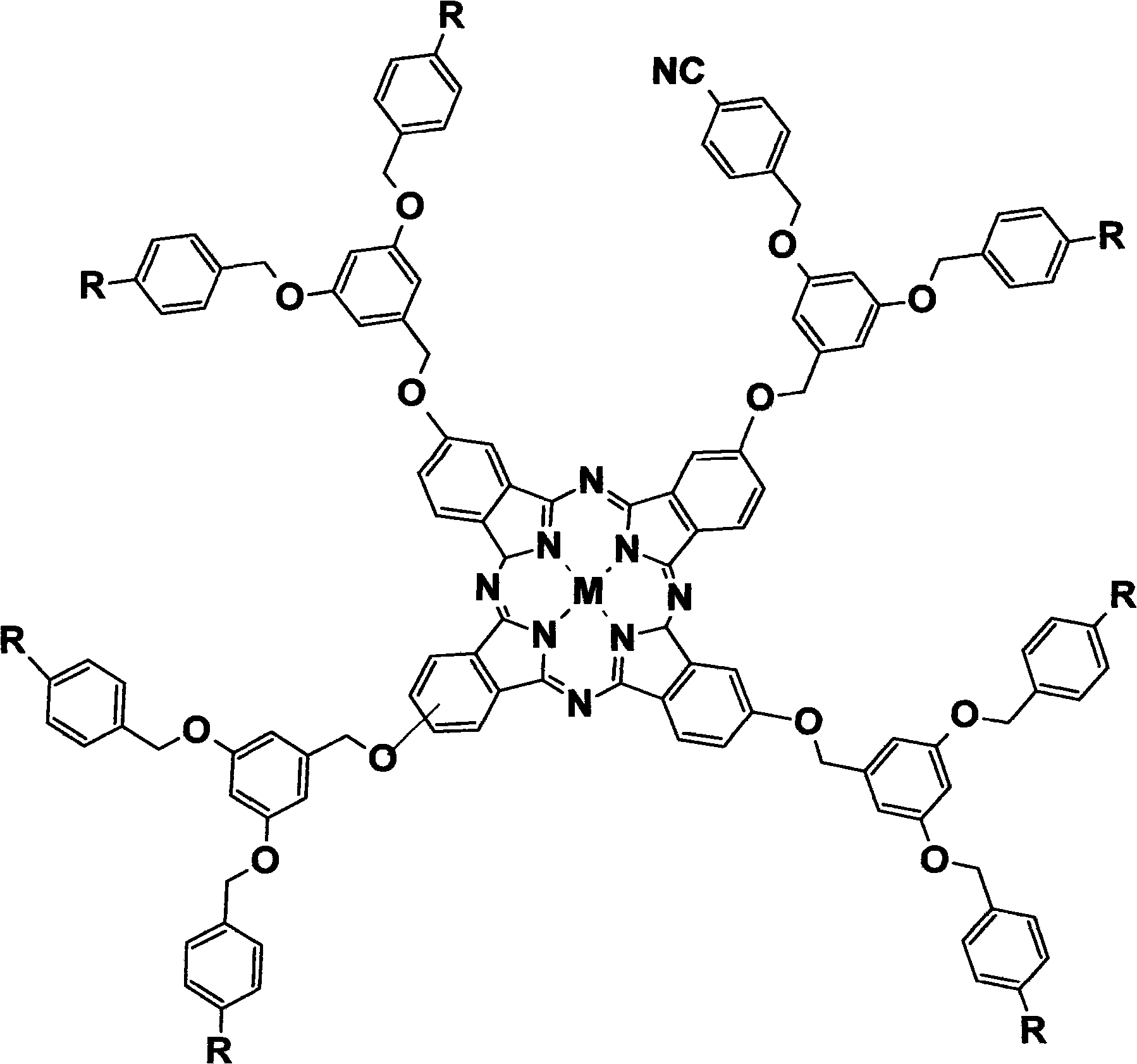
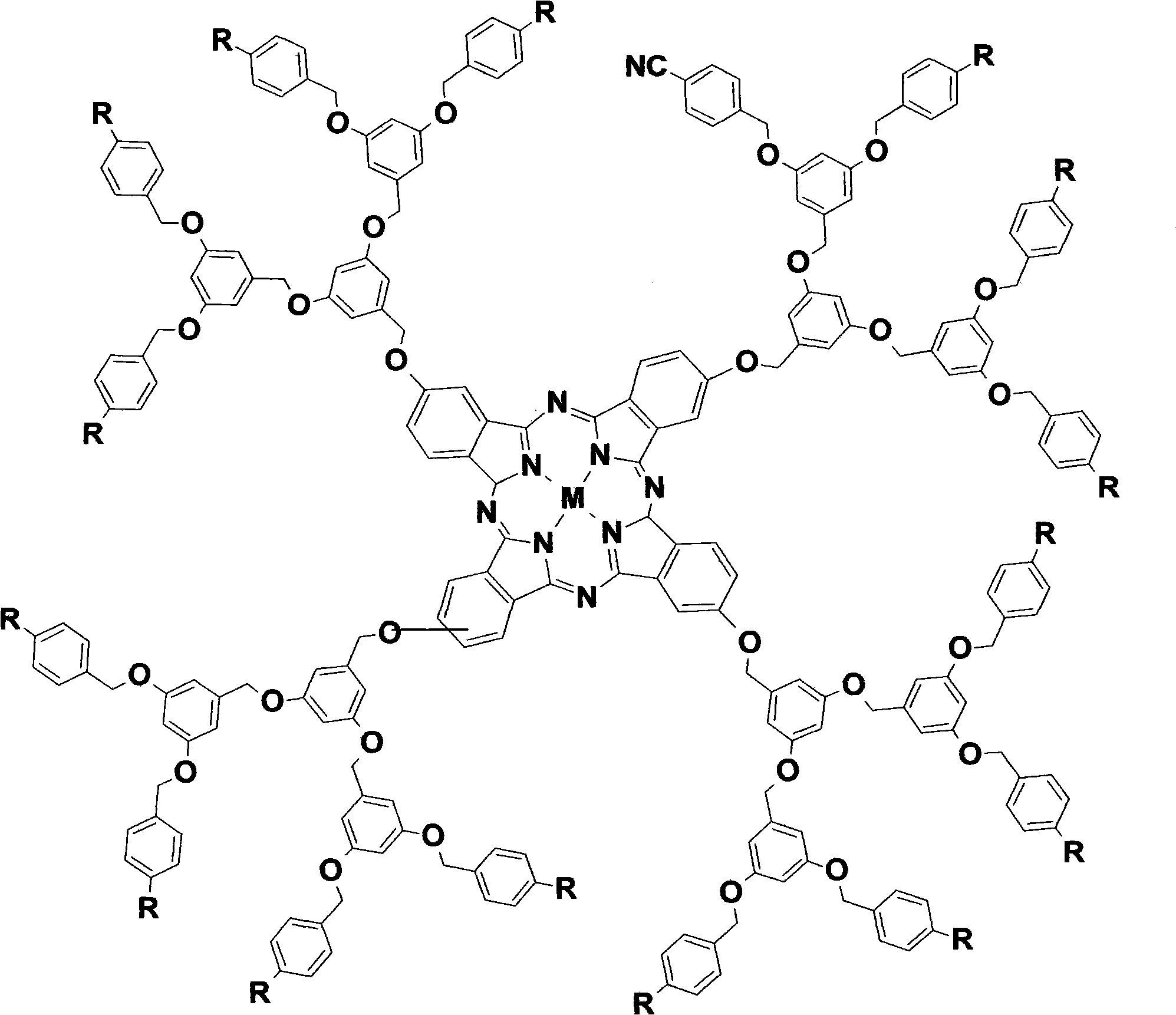
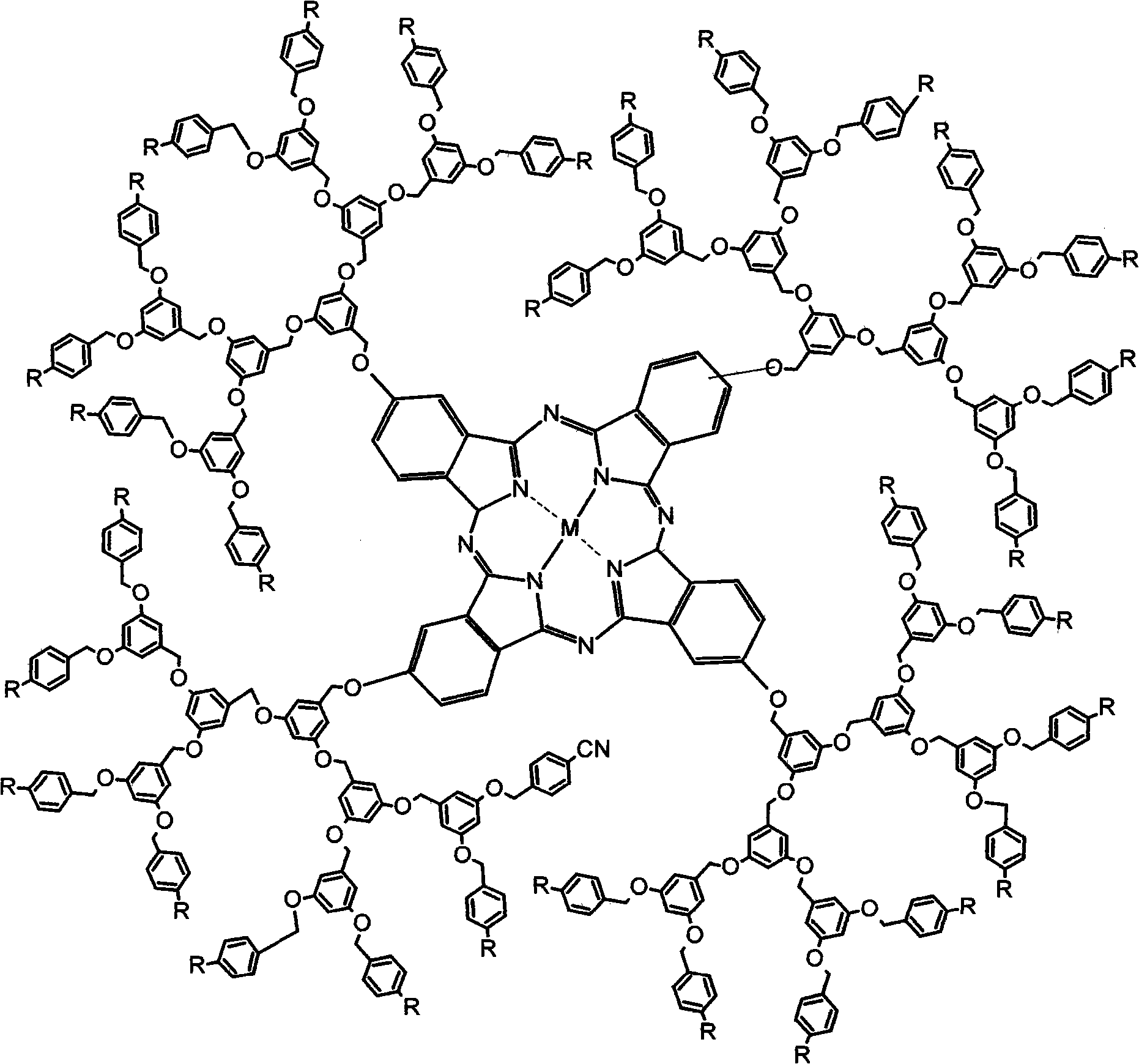
1-3 generation arylene ether dendritic phthalocyanine complex and polymer nano-particle thereof
A nanoparticle and dendritic technology, which is applied in the field of 1-3 generation aryl ether dendritic zinc phthalocyanine complexes and their polymer nanoparticles and preparation fields, can solve the problems of low uptake rate and enrichment effect of new tissues.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] In Example 1, the process 2) carbon tetrabromide was changed to 8g, triphenylphosphine was changed to 4g and tetrahydrofuran was changed to 20mL, and the reaction time was changed to 2h. Other reaction conditions were the same, and 4.0 g of white powdery solid substance (2) was obtained with a yield of 65.0%.
[0090] In process 4), carbon tetrabromide was changed to 15g, triphenylphosphine was changed to 8g and tetrahydrofuran was changed to 35mL, and the reaction time was changed to 2.5h. Other reaction conditions were the same, and 5.04 g of white powdery solid substance (4) was obtained with a yield of 80.0%.
[0091] In process 5), carbon tetrabromide was changed to 30g, triphenylphosphine was changed to 16g and tetrahydrofuran was changed to 60mL, and the reaction time was changed to 3.0h. Other reaction conditions were the same, and 5.25 g of white powdery solid substance (5) was obtained with a yield of 82%.
specific Embodiment 3
[0092] In Example 1, the reaction temperature of process 6) is 80° C., and other reaction conditions are the same, and the yield of the obtained substance (6) is 45.0%.
[0093] Process 7) The reaction temperature is 80°C, other reaction conditions are the same, and the yield of substance (7) is 30.0%.
[0094] Process 8) The reaction temperature is 80°C, other reaction conditions are the same, and the yield of substance (8) is 22.0%.
specific Embodiment 4
[0095] Synthetic method is the same as embodiment one, in process 9)-14), replaces zinc acetate with aluminum trichloride, other process is unchanged, obtains four-[3,5-bis-(4-cyanobenzyloxy)- 1-Methyleneoxyphenyl] aluminum phthalocyanine [G 1 -AlPc(CN) 8 ], productive rate 65%.IR (KBr / cm -1 ): 3449, 2921, 2231, 1601, 1452, 1168, 1059, 826, 640. 1 HNMR (400MHz, DMSO-d 6 , δ / ppm): 7.68(s, 4H), 7.25-7.26(d, J=4Hz, 4H), 7.18~7.19(d, J=4Hz, 4H) 7.66~7.68(d, J=8Hz, 16H) , 7.46~7.48 (d, J=8Hz, 16H), 6.58 (s, 8H), 6.52 (s, 4H), 5.06 (s, 24H). MALDI-TOF-MS: m / z=2068 [M+H 3 o + ]. Four-{3,5-bis-[3,5-bis-(4-cyanobenzyloxy)-1-methoxyphenyl]-1-methoxyphenyl}aluminum phthalocyanine [G 2 -AlPc(CN) 16 ], yield 62%. IR (KBr / cm -1 ): 3450, 2920, 2230, 1600, 1450, 1251, 1153, 1067, 822, 640. 1 H NMR (400MHz, DMSO-d 6 , δ / ppm): 7.81~7.83(d, J=8Hz, 32H), 7.56~7.58(d, J=8Hz, 32H), 7.39(s, 4H), 7.30(s, 4H), 7.20~7.22( d, J=8Hz, 4H), 6.70(s, 16H), 6.61(s, 12H), 6.57(s, 8H), 5.18(s, 40H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com