Fasudil hydrochloride injection composition and its preparation method
A technology for fasudil hydrochloride and injection, which is applied in the field of medicine, can solve the problems of cysteine hydrochloride having a peculiar smell, influence on the quality of preparations, complicated prescriptions, etc. The effect of simple prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
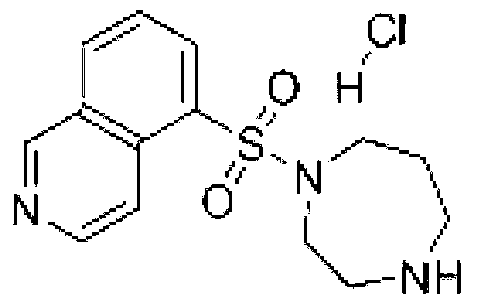
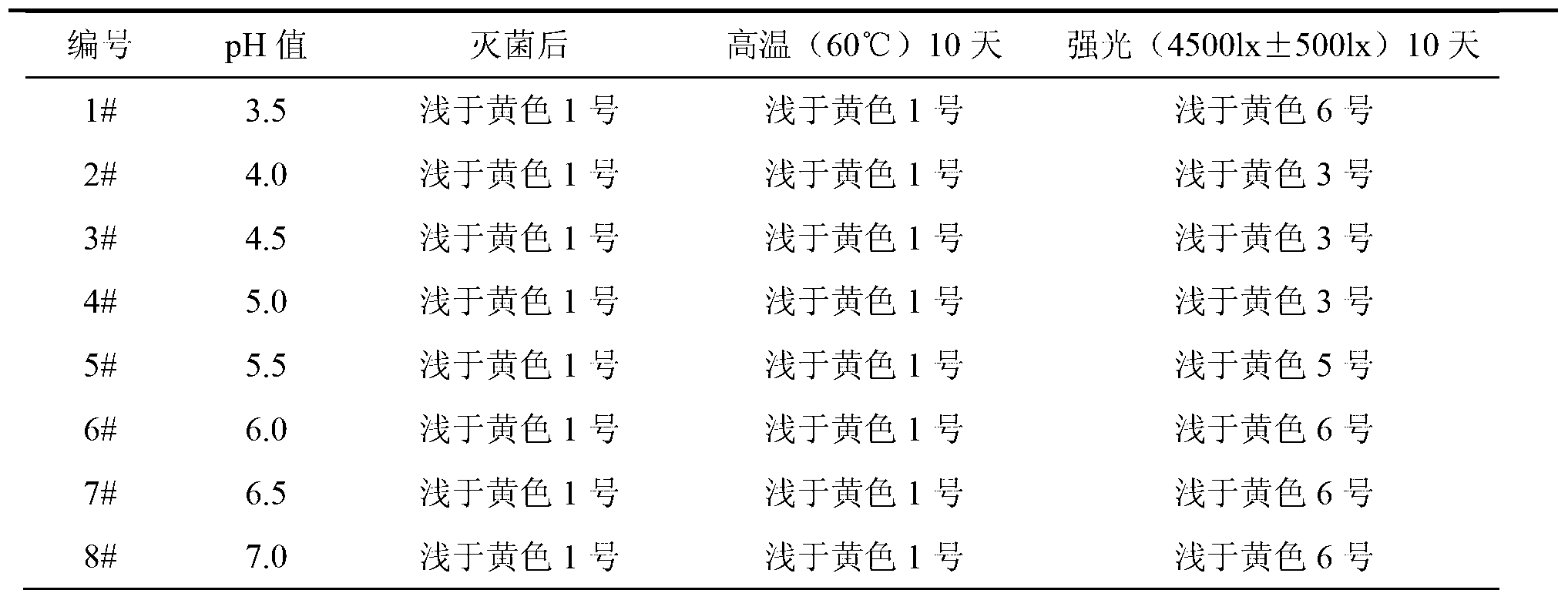
[0058] Weigh 30g of Fasudil hydrochloride and 0.2g of calcium sodium edetate, add 1800ml of water for injection, stir for 15 minutes, dissolve and stir evenly; measure the pH value of the solution, adjust the pH value with 0.1mol / L hydrochloric acid solution 4.5, add water to 2000ml, stir to mix evenly, check the pH value of the solution, if necessary, adjust the pH value to 4.5 with 0.1mol / L hydrochloric acid solution; add 0.1% (W / V) medicinal charcoal, absorb for 20 minutes, and decarbonize; Fine filter with "0.45μm+0.22μm" sterilizing filter in series, fill the filtrate into 2ml colorless and transparent low borosilicate glass ampoule bottle under the condition of nitrogen filling, melt and seal, and extinguish with 115℃ damp heat steam Bacteria for 30 minutes, lamp inspection, to obtain Fasudil Hydrochloride Injection.
Embodiment 2
[0060] Weigh 30g of Fasudil hydrochloride and 0.2g of calcium sodium edetate, add 1600ml of water for injection, stir for 10 minutes, dissolve and stir evenly; measure the pH value of the solution, adjust the pH value with 0.1mol / L hydrochloric acid solution 4.0, add water to 2000ml, stir to mix evenly, check the pH value of the solution, adjust the pH value to 4.0 with 0.1mol / L hydrochloric acid solution if necessary; add 0.1% (W / V) medicinal charcoal, absorb for 20 minutes, and decarbonize; Fine filter with "0.45μm+0.22μm" sterilizing filter in series, fill the filtrate into 2ml colorless and transparent low borosilicate glass ampoule bottle under the condition of nitrogen filling, melt and seal, and extinguish with 115℃ damp heat steam Bacteria for 30 minutes, lamp inspection, to obtain Fasudil Hydrochloride Injection.
Embodiment 3
[0062] Weigh 30g of Fasudil hydrochloride and 0.2g of calcium sodium edetate, add 1700ml of water for injection, stir for 12 minutes, dissolve and stir evenly; measure the pH value of the solution, adjust the pH value with 0.1mol / L hydrochloric acid solution 4.2, add water to 2000ml, stir to mix evenly, check the pH value of the solution, adjust the pH value to 4.2 with 0.1mol / L hydrochloric acid solution if necessary; add 0.1% (W / V) medicinal charcoal, absorb for 20 minutes, and decarbonize; Fine filter with "0.45μm+0.22μm" sterilizing filter in series, fill the filtrate into 2ml colorless and transparent low borosilicate glass ampoule bottle under the condition of nitrogen filling, melt and seal, and extinguish with 115℃ damp heat steam Bacteria for 30 minutes, lamp inspection, to obtain Fasudil Hydrochloride Injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com