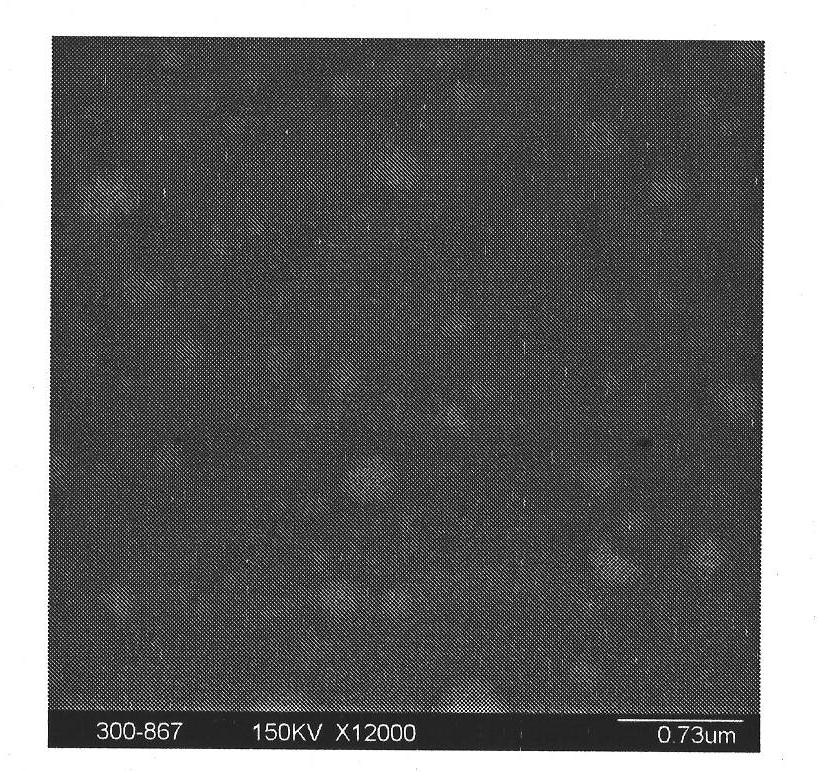
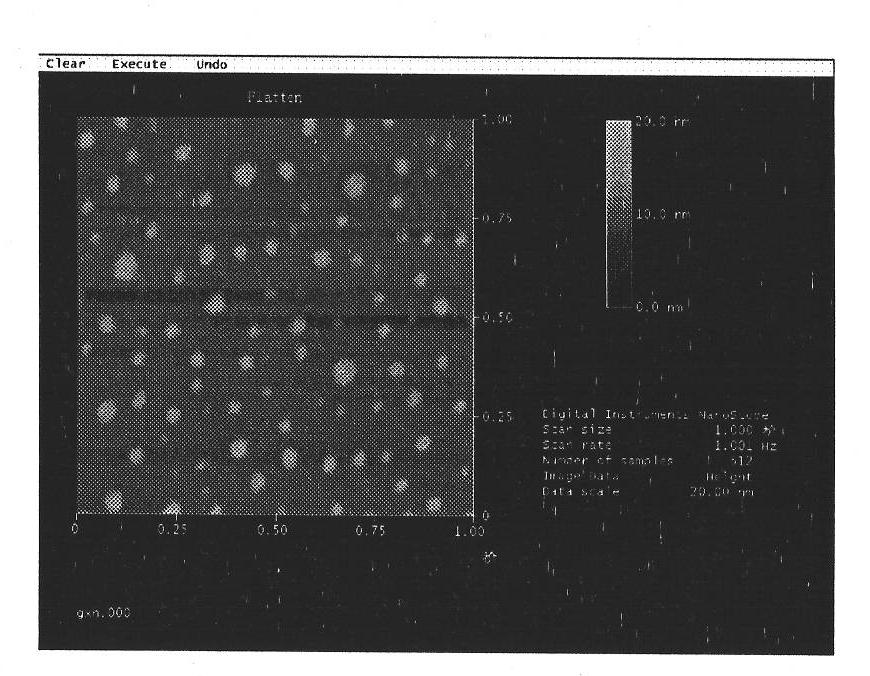
Budesonide nano crystallizing preparation and preparation method thereof
A technology of nano-crystallization and desonide, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor absorption and utilization, improve stability, and improve the preparation process. Ease, facilitate dissolution and absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation method of budesonide nanocrystal, the preparation steps are as follows:
[0023] (1) Disperse 0.5 g of HPMC, 1 g of lecithin and 0.5 g of TPGS in 100 parts by volume of double distilled water as a dispersion medium to obtain solution A;
[0024] (2) 2g of budesonide is dispersed in the aqueous dispersion medium obtained in step (1) to obtain coarse suspension B;
[0025] (3) the coarse suspension B obtained in step (2) is sheared 3min at 15000rpm with a high-speed shear to obtain suspension C;
[0026] (4) adopting the high pressure homogenization method to homogenize the suspension C prepared in step (3) at 500 for 10 times and 1500bar for 20 times to obtain the budesonide nanocrystalline suspension;
[0027] (5) adding 5% (W / V) mannitol to the above-mentioned budesonide nanocrystalline suspension as a freeze-drying protective agent to obtain budesonide crystals;
Embodiment 2
[0029] A preparation method of budesonide nanocrystal, the preparation steps are as follows:
[0030] (1) Disperse PVP 0.5g, Poloxamer F681g, Tween 800.5g in 100 parts by volume of double distilled water as a dispersion medium to obtain solution A;
[0031] (2) 1 g of budesonide is dispersed in the aqueous dispersion medium obtained in step (1) to obtain coarse suspension B;
[0032] (3) the coarse suspension B obtained in step (2) is sheared 2min at 15000rpm with a high-speed shear to obtain suspension C;
[0033] (4) adopting the high pressure homogenization method to homogenize the suspension C prepared in step (3) at 600 for 10 times and 10 times at 1800 bar to obtain the budesonide nanocrystalline suspension;
[0034] (5) adding 10% (W / V) mannitol to the above-mentioned budesonide nanocrystalline suspension as a freeze-drying protective agent to obtain budesonide crystals;
[0035] Application of budesonide nanocrystalline suspension in drug delivery system:
[0036] 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com