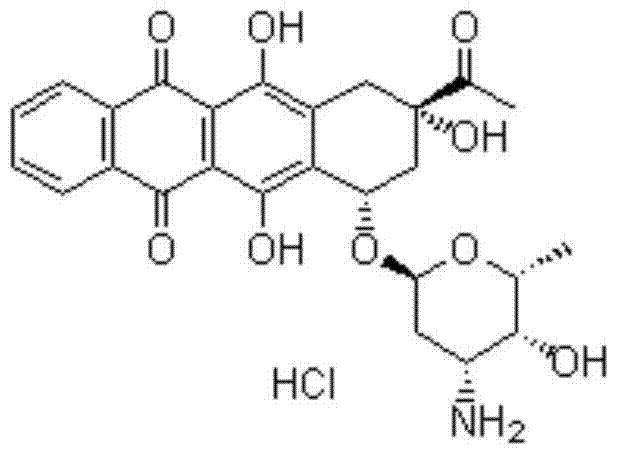
Idarubicin hydrochloride pharmaceutical composition and preparation method thereof
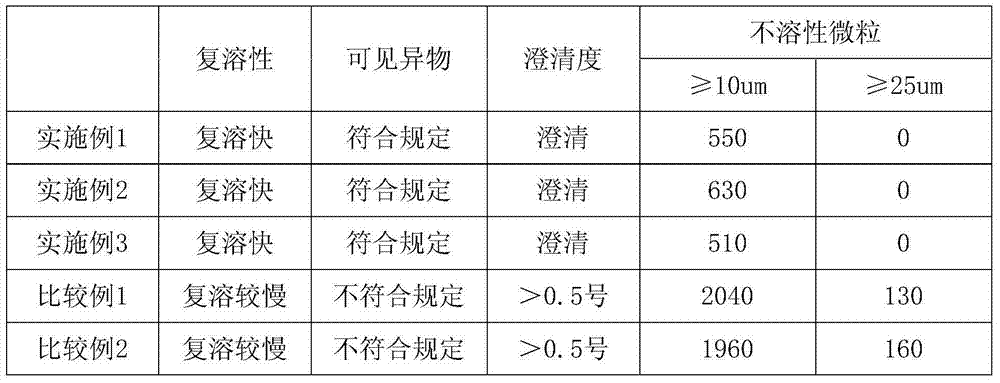
A technology for idarubicin hydrochloride and its composition, which is applied in the field of idarubicin hydrochloride composition and its preparation, can solve the problems of insoluble particles, unqualified clarity, incomplete dissolution, and foreign matter, and achieve considerable economic and Social benefits, improved reconstitution performance, uniform and accurate content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The described idarubicin hydrochloride pharmaceutical composition of every 1000 bottles, its formula consists of:
[0059] Idarubicin Hydrochloride 10g
[0060] Lactose 50g
[0061] Add purified water to 50kg
[0062] Preparation Process:
[0063] (1) Preparation: Put idarubicin hydrochloride and lactose in the proportion of 1:1 to 20 by weight in a liquid preparation tank, add water for injection, stir to dissolve completely and mix evenly;
[0064] (2) Sterile filtration and sub-packaging: filter the idarubicin hydrochloride solution obtained above through a microporous membrane into a sterile room, sub-pack into vials, and half stopper;
[0065] (3) Vacuum freeze drying:
[0066] a. Pre-freezing: Put the above-mentioned subpackaged idarubicin hydrochloride liquid into a freeze dryer, reduce the temperature of the product at a rate of 10-15°C / hour, and keep it warm for 60 minutes when the temperature of the product is lower than –40°C. The idarubicin hydrochloride ...
Embodiment 2
[0078] The described idarubicin hydrochloride pharmaceutical composition of every 1000 bottles, its formula consists of:
[0079] Idarubicin Hydrochloride 5g
[0080] Lactose 50g
[0081] Add purified water to 50kg
[0082] Preparation process: with embodiment 1.
Embodiment 3
[0084] The described idarubicin hydrochloride pharmaceutical composition of every 1000 bottles, its formula consists of:
[0085] Idarubicin Hydrochloride 5g
[0086] Lactose 30g
[0087] Add purified water to 50kg
[0088] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com