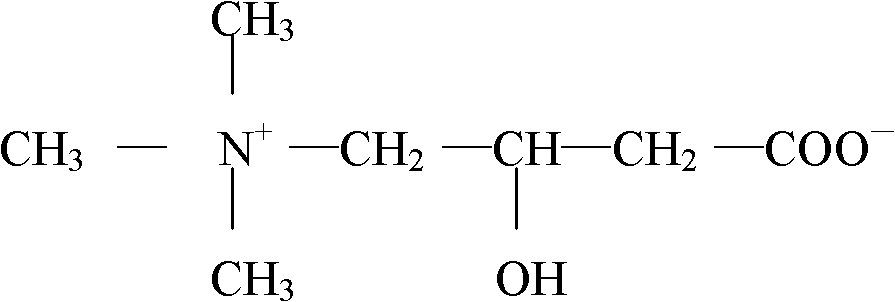
Levocarnitine composition for injection and preparation method of levocarnitine composition
A technology for composition and injection, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of decarbonization and filtration of medicinal liquids, and improve the efficiency of freeze-drying , increase the lower limit temperature, and solve the effect of poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] prescription:
[0087] Levocarnitine 10Kg
[0088] Mannitol 10Kg
[0089] Add water for injection to 80Kg
[0090] Preparation: Weigh levocarnitine and mannitol, put them in a preparation tank, add water for injection, stir to dissolve completely and mix evenly, and adjust the pH to 6.0 with 0.1mol / L hydrochloric acid solution;
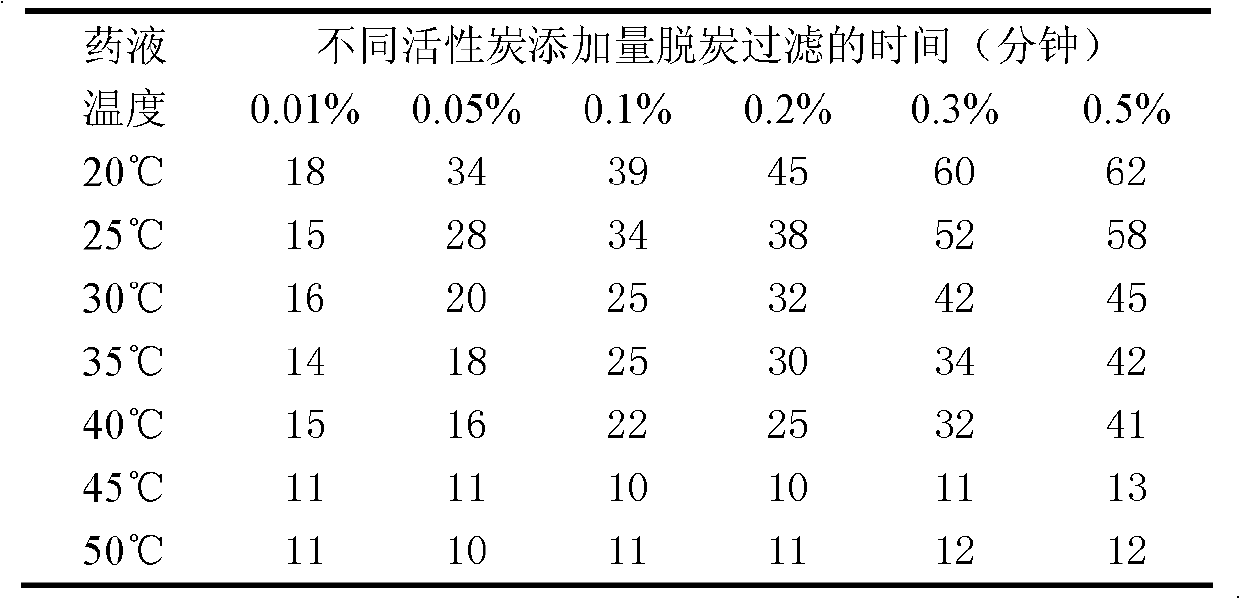
[0091] Decarbonization and sterile filtration: Add 0.3% activated carbon for needles by weight of the liquid medicine, continue to stir, heat the liquid medicine to 45°C and keep it warm for 30 minutes, connect a titanium rod filter and a 0.22 μm microporous filter in series, and decarbonize Simultaneously carry out sterile filtration;
[0092] Aseptic subpackaging: subpackage the levocarnitine solution filtered into the sterile room into 8ml / bottles into vials, and half-stopper;
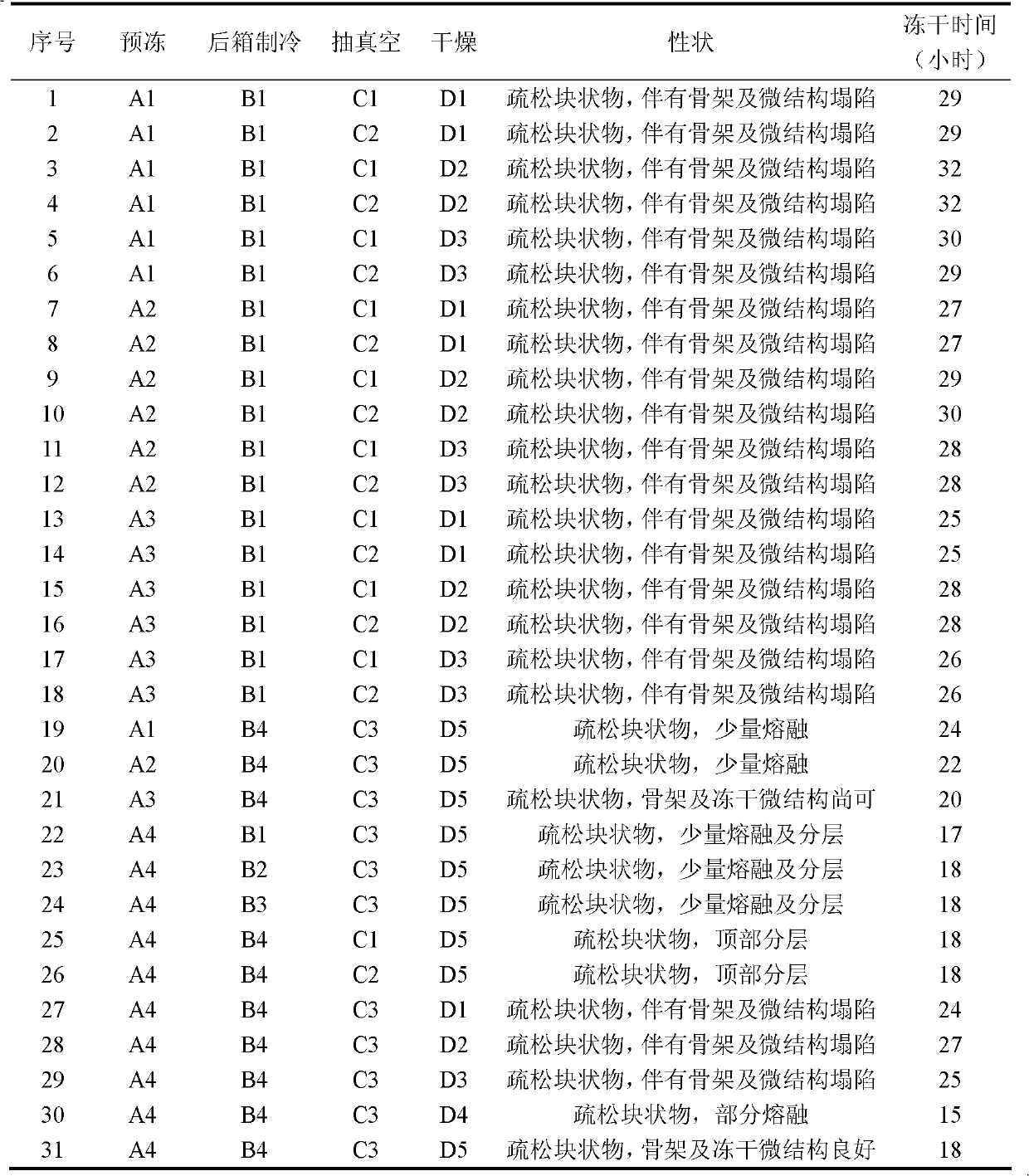
[0093] Vacuum Freeze Drying:
[0094] a. Pre-freezing: Put the sub-packaged levocarnitine liquid into the freeze dryer, quickly lower the product temperature, a...
Embodiment 2
[0101] prescription:
[0102] Levocarnitine 10Kg
[0103] Mannitol 11Kg
[0104] Add water for injection to 80Kg
[0105] Preparation: Weigh levocarnitine and mannitol, put them in a preparation tank, add water for injection, stir to dissolve completely and mix evenly, and adjust the pH to 6.3 with 0.1mol / L hydrochloric acid solution;
[0106] Decarbonization and sterile filtration: Add 0.3% activated carbon for needles by weight of the liquid medicine, continue to stir, heat the liquid medicine to 45°C and keep it warm for 30 minutes, connect a titanium rod filter and a 0.22 μm microporous filter in series, and decarbonize Simultaneously carry out sterile filtration;
[0107] Aseptic subpackaging: subpackage the levocarnitine solution filtered into the sterile room into 8ml / bottles into vials, and half-stopper;
[0108] Vacuum Freeze Drying:
[0109] a. Pre-freezing: Put the sub-packaged levocarnitine liquid into the freeze dryer, quickly lower the product temperature, a...
Embodiment 3
[0116] prescription:
[0117] Levocarnitine 10Kg
[0118] Mannitol 7.5Kg
[0119] Add water for injection to 80Kg
[0120] Preparation: Weigh levocarnitine and mannitol, put them in a preparation tank, add water for injection, stir to dissolve completely and mix evenly, and adjust the pH to 6.1 with 0.1mol / L hydrochloric acid solution;
[0121] Decarbonization and sterile filtration: Add 0.3% activated carbon for needles by weight of the liquid medicine, continue to stir, heat the liquid medicine to 45°C and keep it warm for 30 minutes, connect a titanium rod filter and a 0.22 μm microporous filter in series, and decarbonize Simultaneously carry out sterile filtration;
[0122] Aseptic subpackaging: subpackage the levocarnitine solution filtered into the sterile room into 8ml / bottles into vials, and half-stopper;
[0123] Vacuum Freeze Drying:
[0124] a. Pre-freezing: Put the sub-packaged levocarnitine liquid into the freeze dryer, quickly lower the product temperature, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com