A kind of ulinastatin freeze-dried powder preparation and preparation method thereof
A technology of dry powder preparation and ulinastatin, which is applied in the field of ulinastatin freeze-dried powder preparation and its preparation, can solve problems such as unsatisfactory stability, affecting activity, affecting drug efficacy, etc., and achieve reconstitution performance and stability Excellent, prevents failure, and accelerates the drying rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
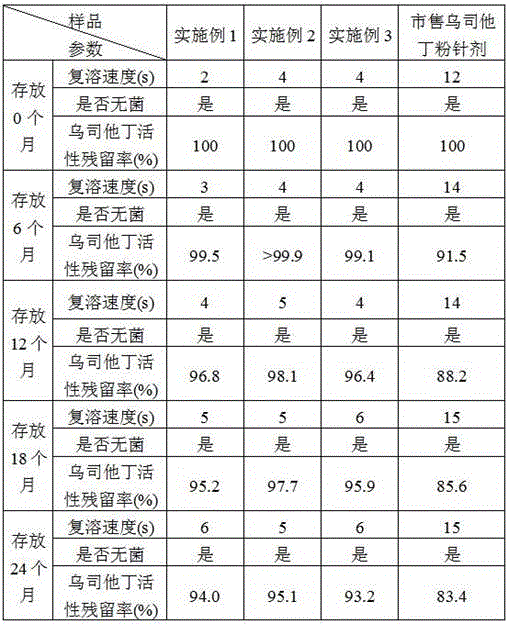
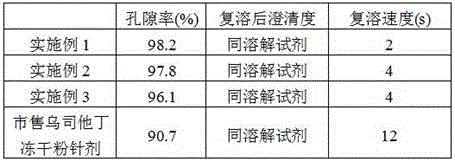
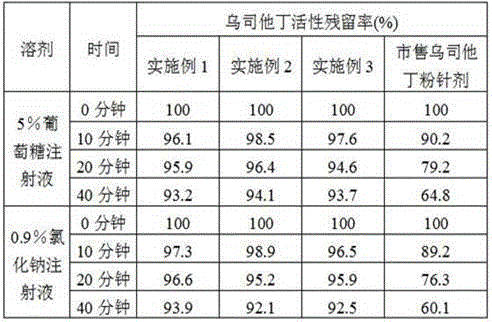
[0042] Embodiment 1: A kind of ulinastatin freeze-dried powder preparation
[0043] The formula of the ulinastatin freeze-dried powder preparation is, per 1000 vials: 25 million units of ulinastatin, 1 g of mannitol, 0.17 g of sodium dihydrogen phosphate, and 0.32 g of disodium hydrogen phosphate.
[0044] The preparation method of described ulinastatin freeze-dried powder preparation is:
[0045] ⑴. Weigh mannitol, sodium chloride, disodium hydrogen phosphate or sodium dihydrogen phosphate in the liquid mixing tank according to the dosage of the formula, stir with water for injection at a temperature of 2°C until dissolved; add medical activated carbon with an average particle size of 2 μm and stir After mixing, use a 0.45 μm filter membrane to filter decarburization twice, and keep the temperature at 2°C. This solution is the excipient solution; dissolve ulinastatin with water for injection at 2°C, and mix it with the above-mentioned excipient solution at 2°C. To obtain the...
Embodiment 2
[0055] Embodiment 2: a kind of ulinastatin freeze-dried powder preparation
[0056] The formula of the ulinastatin freeze-dried powder preparation is, per 1000 vials: 50 million units of ulinastatin, 5 g of mannitol, 1 g of sodium chloride, 0.34 g of sodium dihydrogen phosphate, and 0.64 g of disodium hydrogen phosphate.
[0057] The preparation method of described ulinastatin composition freeze-dried powder injection is:
[0058] ⑴. Weigh mannitol, sodium chloride, disodium hydrogen phosphate or sodium dihydrogen phosphate in the liquid mixing tank according to the dosage of the above formula, stir with water for injection at a temperature of 3°C until dissolved; add medical activated carbon with an average particle size of 4 μm After stirring and mixing, use a 0.45μm filter membrane to filter decarburization twice, and keep the temperature at 3°C. This solution is the excipient solution; dissolve ulinastatin with 3°C water for injection, and mix well with the above 3°C excip...
Embodiment 3
[0068] Embodiment 3: A kind of ulinastatin freeze-dried powder preparation
[0069] The formula of the ulinastatin freeze-dried powder preparation is, per 1000 vials: 100 million units of ulinastatin, 10 g of mannitol, 5 g of sodium chloride, 0.51 g of sodium dihydrogen phosphate, and 0.93 g of disodium hydrogen phosphate.
[0070] The preparation method of described ulinastatin freeze-dried powder preparation is:
[0071] ⑴. Weigh mannitol, sodium chloride, disodium hydrogen phosphate or sodium dihydrogen phosphate in the liquid mixing tank according to the dosage of the above formula, stir with water for injection at a temperature of 4°C until dissolved; add medical activated carbon with an average particle size of 5 μm After stirring and mixing, use a 0.45μm filter membrane to filter decarburization twice, and keep the temperature at 4°C. This solution is the excipient solution; dissolve ulinastatin with 4°C water for injection, and mix well with the above 4°C excipient sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com