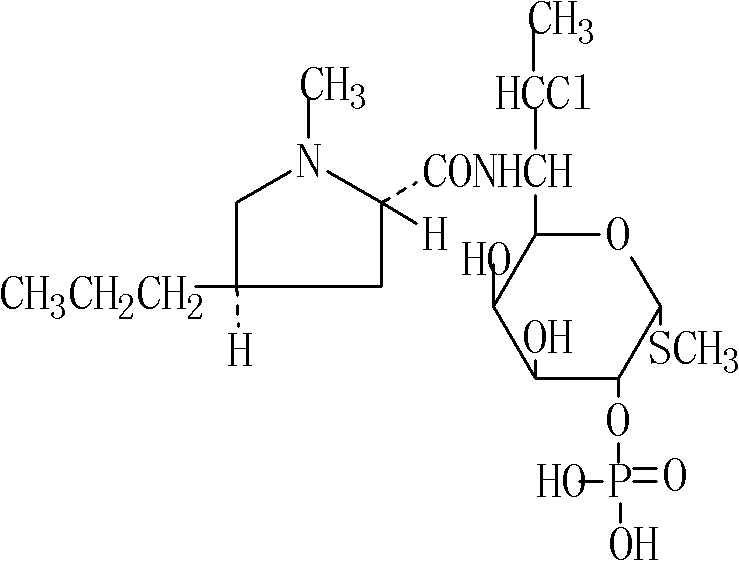
Clindamycin phosphate composition for injection and preparation method thereof
A technology of clindamycin phosphate and composition, which is applied in the field of clindamycin phosphate composition for injection and its preparation, can solve the problems of decarbonization and filtration difficulties, and achieve the effect of excellent freeze-dried structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] prescription:
[0090] Clindamycin Phosphate 3660g
[0091] Sodium hydroxide 146.4g
[0092] Add water for injection to 20Kg
[0093] Preparation: Weigh clindamycin phosphate and sodium hydroxide, put them in a preparation tank, add water for injection, stir to dissolve completely and mix evenly;
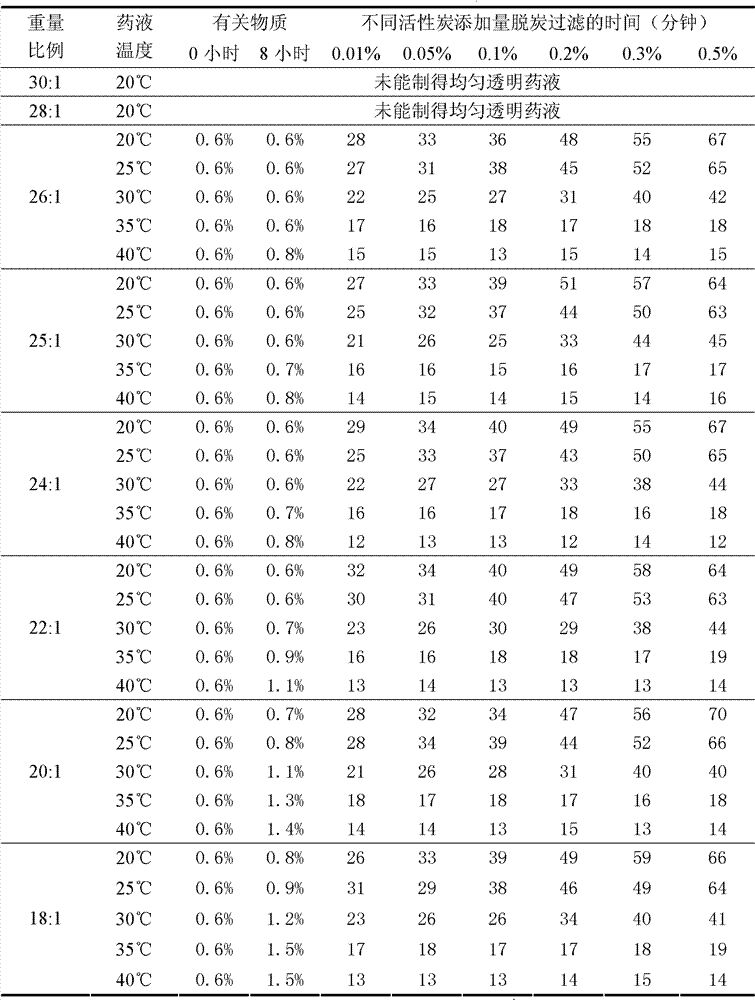
[0094] Decarbonization and sterile filtration: Add 0.3% activated carbon for needles by weight of the liquid medicine, continue to stir, heat the liquid medicine to 35°C and keep it warm for 20 minutes, connect a titanium rod filter and a 0.22 μm microporous filter in series, and decarbonize Simultaneously carry out sterile filtration;
[0095] Sterile packaging: the clindamycin phosphate solution filtered into the sterile room is divided into 2 ml / bottle into vials, and half stoppered;
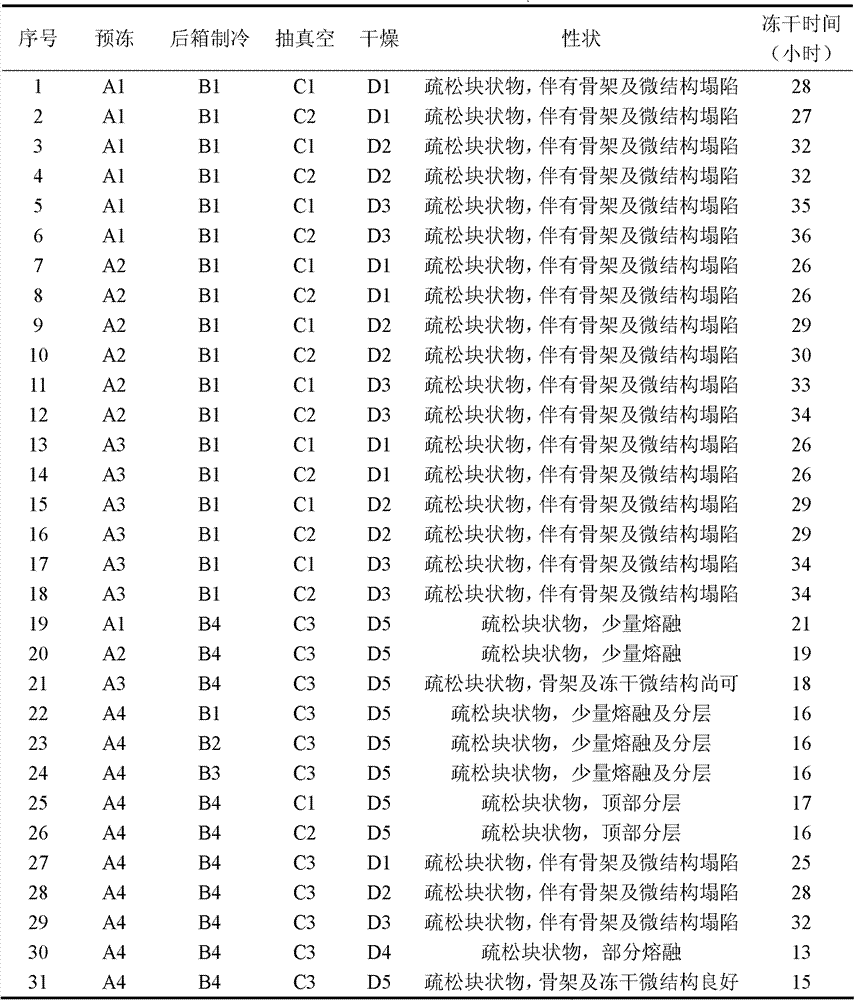
[0096] Vacuum Freeze Drying:
[0097] a. Pre-freezing: Put the subpackaged clindamycin phosphate liquid in a freeze dryer to rapidly reduce the product temperature. When the product tem...
Embodiment 2
[0104] prescription:
[0105] Clindamycin Phosphate 3660g
[0106] Sodium hydroxide 140.8g
[0107] Add water for injection to 20Kg
[0108] Preparation: Weigh clindamycin phosphate and sodium hydroxide, put them in a preparation tank, add water for injection, stir to dissolve completely and mix evenly;
[0109] Decarbonization and sterile filtration: Add 0.3% activated carbon for needles by weight of the liquid medicine, continue to stir, heat the liquid medicine to 35°C and keep it warm for 20 minutes, connect a titanium rod filter and a 0.22 μm microporous filter in series, and decarbonize Simultaneously carry out sterile filtration;
[0110] Sterile packaging: the clindamycin phosphate solution filtered into the sterile room is divided into 2 ml / bottle into vials, and half stoppered;
[0111] Vacuum Freeze Drying:
[0112] a. Pre-freezing: Put the subpackaged clindamycin phosphate liquid in a freeze dryer to rapidly reduce the product temperature. When the product tem...
Embodiment 3
[0119] prescription:
[0120] Clindamycin Phosphate 3660g
[0121] Sodium hydroxide 152.5g
[0122] Add water for injection to 20Kg
[0123] Preparation: Weigh clindamycin phosphate and sodium hydroxide, put them in a preparation tank, add water for injection, stir to dissolve completely and mix evenly;
[0124] Decarbonization and sterile filtration: Add 0.3% activated carbon for needles by weight of the liquid medicine, continue to stir, heat the liquid medicine to 35°C and keep it warm for 20 minutes, connect a titanium rod filter and a 0.22 μm microporous filter in series, and decarbonize Simultaneously carry out sterile filtration;
[0125] Sterile packaging: the clindamycin phosphate solution filtered into the sterile room is divided into 2 ml / bottle into vials, and half stoppered;
[0126] Vacuum Freeze Drying:
[0127] a. Pre-freezing: Put the subpackaged clindamycin phosphate liquid in a freeze dryer to rapidly reduce the product temperature. When the product tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com