A kind of lansoprazole freeze-dried agent for injection and preparation method thereof
A technology of lansoprazole freeze-dried agent and lansoprazole, applied in freeze-dried transportation, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of affecting compatibility stability and reducing product Safety, insoluble particles and other issues, to achieve the effect of improving stability and resolubility, short freeze-drying time, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides the preparation method of above-mentioned lansoprazole freeze-dried preparation for injection, comprises the following steps:
[0042] S1. Dosing: add water for injection to the formulated amount of lansoprazole, pullulan, histidine and hydroxypropyl-β-cyclodextrin, stir and dissolve, and use a concentration of 0.2-0.3mol / L The sodium hydroxide solution adjusted pH value is 10-11, obtains solution;
[0043] S2. Sterilization and filtration: pass the solution obtained in step S1 with a concentration of 16-20 mg / ml through a 0.22 μm filter membrane,
[0044] Sterilize by filtration to obtain a sterile solution;
[0045]S3. Filling and half-tamping: filling the aseptic solution obtained in step S2 into a vial, and half-tightening to obtain a sample solution;
[0046] S4. Freeze-drying: freeze-dry the sample solution obtained in step S3 to obtain a freeze-dried sample;
[0047] The freeze-drying method is as follows: cool the sample li...
Embodiment 1-9
[0051] The preparation method of embodiment 1-9, comprises the following steps:
[0052] S1. Dosing: Add water for injection to the formulated amount of lansoprazole, pullulan, histidine and hydroxypropyl-β-cyclodextrin, stir and dissolve, and use hydrogen with a concentration of 0.2mol / L The sodium oxide solution adjusts the pH value to 10 to obtain a solution;
[0053] S2. Sterilization and filtration: pass the solution obtained in step S1 with a concentration of 18 mg / ml through a 0.22 μm filter membrane,
[0054] Sterilize by filtration to obtain a sterile solution;
[0055] S3. Filling and half-tamping: filling the aseptic solution obtained in step S2 into a vial, and half-tightening to obtain a sample solution;
[0056] S4. Freeze-drying: Freeze-dry the sample liquid obtained in step S3, cool the sample liquid at 27°C to -42°C within 0.5h, and keep it for 0.5h, then raise the temperature to -5°C within 1h, and keep it for 4h, Raise the temperature to 0°C within 12 min...
experiment example 1
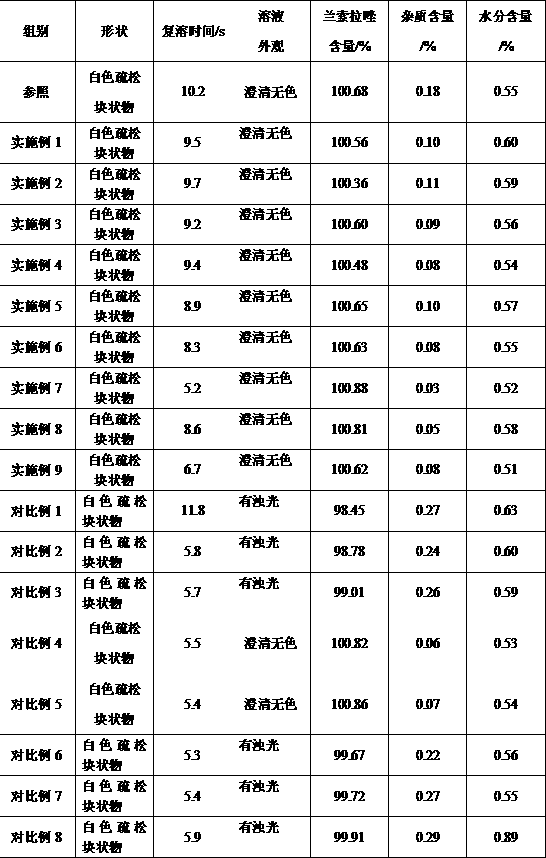
[0087] Experimental example 1, basic performance
[0088] The lansoprazole freeze-dried powder for injection that each embodiment and comparative example make carries out basic performance detection, adopts observation method to observe the outward appearance character of lansoprazole freeze-dried powder, freeze-dried powder is dissolved in the water for injection Make the injection that concentration is 3.5mg / mL, observe the color of injection, clarity and reconstitution time, and according to the high performance liquid chromatography of Chinese Pharmacopoeia 2010 edition two appendix V D, to lansoprazole freeze-drying Lansoprazole content, impurity content in the powder are measured; According to the moisture determination method of the Chinese Pharmacopoeia 2010 edition two appendix VIII M first method, the moisture in the lansoprazole freeze-dried powder is measured. Simultaneously, buy the freeze-dried powder injection of trade name " lansoprazole for injection " produce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com