Potassium magnesium aspartate freeze-dried powder preparation for injection and preparation method of preparation
A technology of potassium magnesium aspartate freeze-dried powder and magnesium aspartate, which is applied in the field of medicine, can solve the problem that the stability and resolubility of the preparation need to be improved, the amount of excipients affects the freeze-drying of the product, and the medicinal effect of the powder injection is general, etc. problems, to achieve the effect of improving resolubility, scientific and reasonable preparation methods, and avoiding curative effect problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
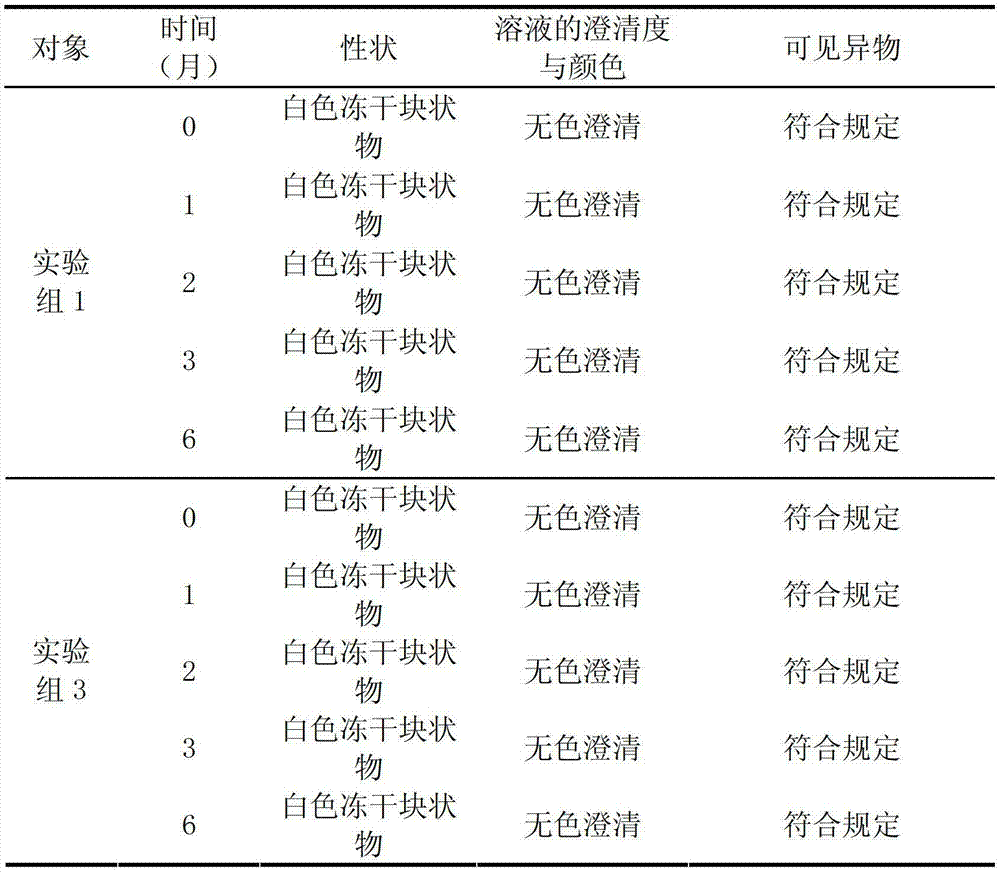
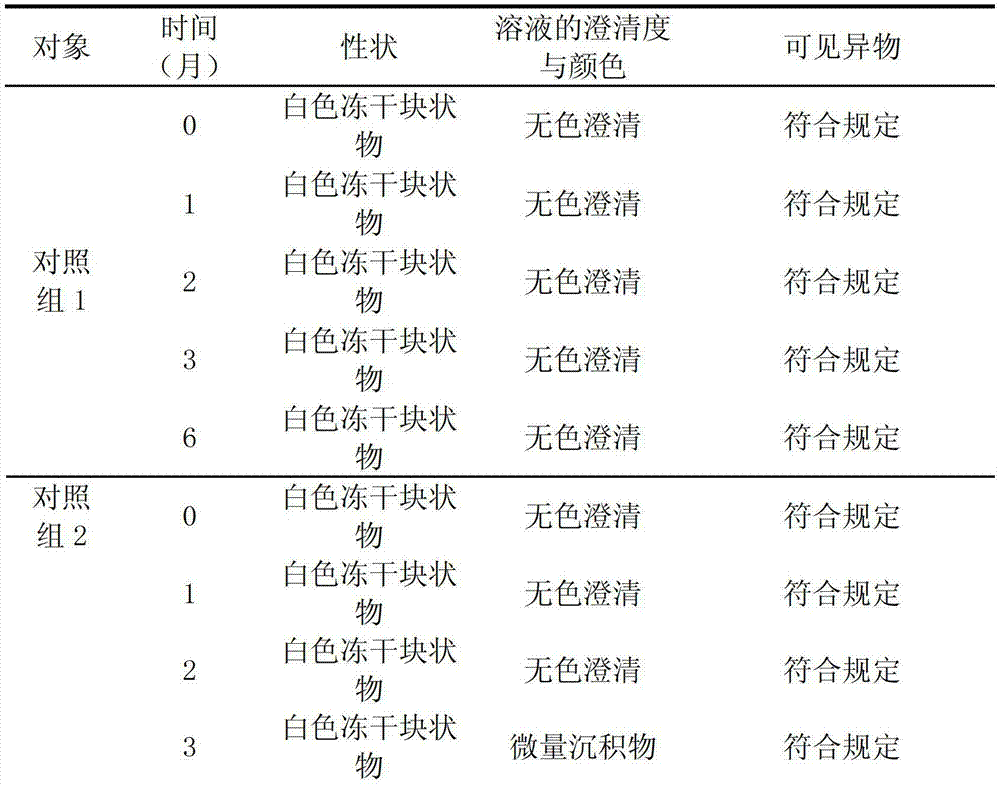
Examples
Embodiment 1
[0046] Prescription: 1000 bottles are prepared from the following raw materials: potassium aspartate 500g, magnesium aspartate 500g and lactose 400g, add water for injection to 12L;
[0047] Preparation:
[0048] (1) Add 60% of the prescription water for injection at 70°C to the concentrated preparation tank, slowly add the prescription amount of potassium aspartate, magnesium aspartate and lactose into the tank in turn, stir to dissolve, and add water for injection to the full amount;
[0049] (2) After the dissolution is complete, take a sample to measure the pH value, and adjust the pH value to 6.0 with a pH regulator;
[0050] (3) Add the wetted activated carbon with a weight-to-volume ratio of 0.1% into the liquid preparation tank, control the liquid temperature at 58 °C and stir for 30 minutes, and decarbonize and filter through a 0.45 μm microporous membrane;
[0051] (4) Filter the solution from the concentrated preparation tank to the dilute preparation tank; test t...
Embodiment 2
[0056] Prescription: 1000 bottles are prepared from the following raw materials: potassium aspartate 1000g, magnesium aspartate 1000g and lactose 800g, add water for injection to 25L;
[0057] Preparation:
[0058] (1) Add 60% of the prescription water for injection at 70°C to the concentrated preparation tank, slowly add the prescription amount of potassium aspartate, magnesium aspartate and lactose into the tank in turn, stir to dissolve, and add water for injection to the full amount;
[0059] (2) After the dissolution is complete, take a sample to measure the pH value, and adjust the pH value to 6.0 with a pH regulator;
[0060] (3) Add the wetted activated carbon with a weight-to-volume ratio of 0.1% into the liquid preparation tank, control the liquid temperature at 58 °C and stir for 30 minutes, and decarbonize and filter through a 0.45 μm microporous membrane;
[0061] (4) Filter the solution from the concentrated preparation tank to the dilute preparation tank; test...
Embodiment 3
[0066] Prescription: 1000 bottles are prepared from the following raw materials: potassium aspartate 450g, magnesium aspartate 450g and lactose 360g, add water for injection to 9L;
[0067] Preparation:
[0068] (1) Add 50% water for injection at 60°C in the concentrated dispensing tank, slowly add potassium aspartate, magnesium aspartate and lactose in the prescribed amount into the tank, stir and dissolve, and add water for injection to the full amount;
[0069] (2) After the dissolution is complete, take a sample to measure the pH value, and adjust the pH value to 5.0 with a pH regulator;
[0070] (3) Add the wetted activated carbon with a weight-to-volume ratio of 0.1% into the dosing tank, control the liquid temperature at 55°C and stir for 20 minutes, and decarbonize and filter through a 0.45μm microporous membrane;
[0071] (4) Filter the solution from the concentrated preparation tank to the dilute preparation tank; test the intermediate; after passing the test, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com