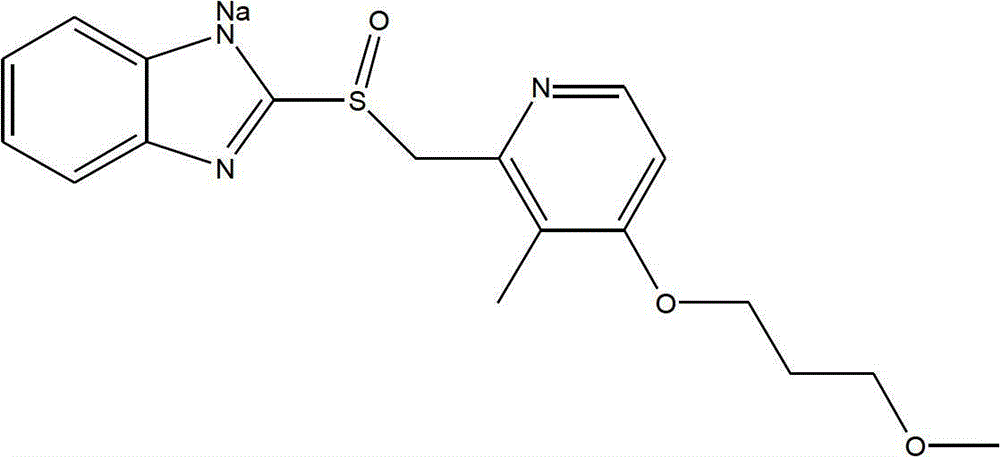
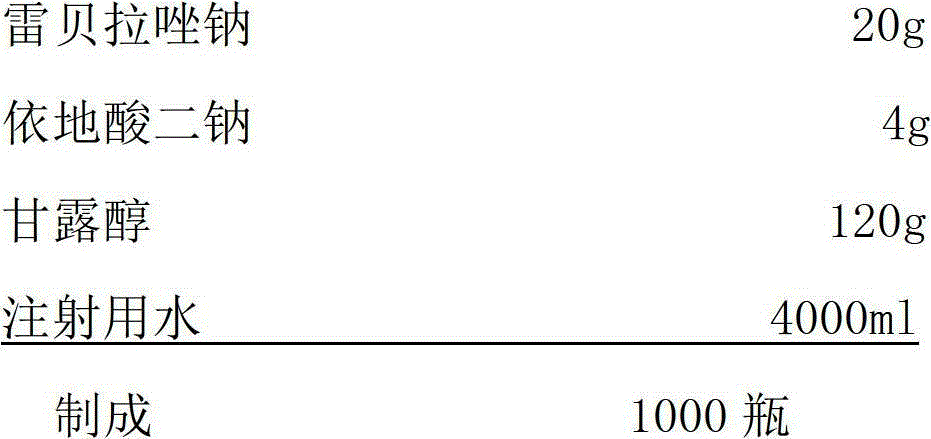Sodium rabeprazole composition for injection
A technology of rabeprazole sodium and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of low production efficiency, shortened freeze-drying time, and long freeze-drying time, and achieve low production cost, shortened freeze-drying time, and low water content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] prescription:
[0089]
[0090] Weigh rabeprazole sodium and edetate disodium into the preparation tank, add water for injection, stir to dissolve and mix evenly, adjust the pH value of the solution to 10.5~12.5 with sodium hydroxide; use a 0.22um filter for the prepared medicinal solution Sterilize and filter to a sterile room, dispense into vials, and half stoppered with rubber. The product was first pre-frozen to -45°C, kept warm for 2 hours, started vacuuming, raised the temperature to 0°C, kept for 4 hours, then raised to 40°C, kept for 5 hours, and kept at 30°C for 3 hours to obtain the composition needle.
Embodiment 2
[0092] prescription:
[0093]
[0094] Weigh rabeprazole sodium and edetate disodium into the preparation tank, add water for injection, stir to dissolve and mix evenly, adjust the pH value of the solution to 10.5~12.5 with sodium hydroxide; use a 0.22um filter for the prepared medicinal solution Sterilize and filter to a sterile room, dispense into vials, and half stoppered with rubber. The product was first pre-frozen to -45°C, kept warm for 2 hours, started vacuuming, raised the temperature to 0°C, kept for 4 hours, then raised to 40°C, kept for 5 hours, and kept at 30°C for 3 hours to obtain the composition needle.
Embodiment 3
[0096] prescription:
[0097]
[0098] Weigh rabeprazole sodium and edetate disodium into the preparation tank, add water for injection, stir to dissolve and mix evenly, adjust the pH value of the solution to 10.5~12.5 with sodium hydroxide; use a 0.22um filter for the prepared medicinal solution Sterilize and filter to a sterile room, dispense into vials, and half stoppered with rubber. The product was first pre-frozen to -45°C, kept warm for 2 hours, started vacuuming, raised the temperature to 0°C, kept for 4 hours, then raised to 40°C, kept for 5 hours, and kept at 30°C for 3 hours to obtain the composition needle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com