Selective reduction method for nitro
A selective, nitro-based technology, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve problems such as difficult separation, strong taste, and slow addition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
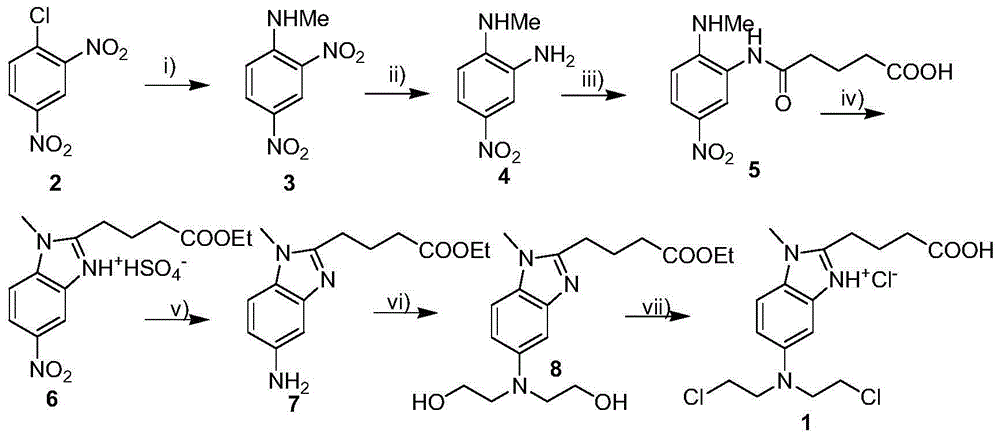
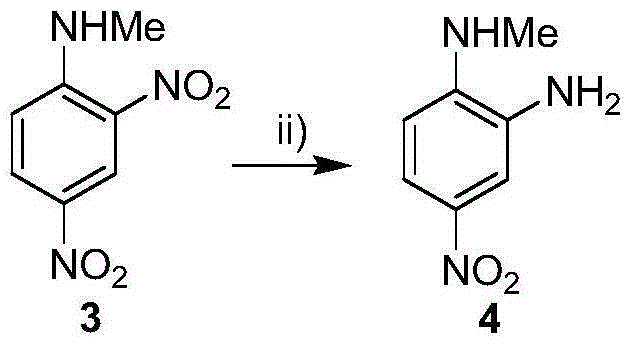
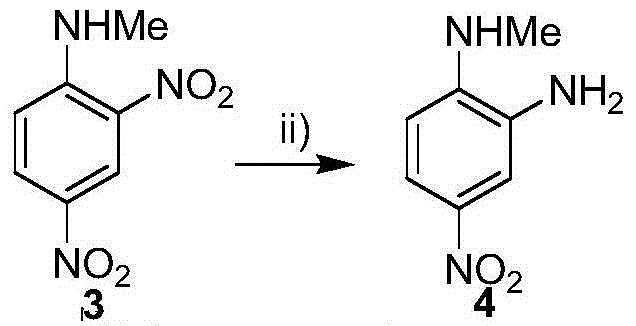
[0009] Embodiment 1: the synthesis of 2-methylamino-5-nitroaniline (compound 4)
[0010]
[0011] 5.0 g of 2,4-dinitro-N-methylaniline (compound 3) was dissolved in 30 mL of acetonitrile, and 0.5 g of palladium carbon (water content 48%) was added, then the temperature was lowered to -10°C, and formic acid was added dropwise to the acetonitrile Solution (5mL of formic acid was added to 10mL of acetonitrile to configure). After the dropwise addition was completed, the temperature was raised to reflux for 3 hours, and the filtrate was collected by filtration. The filtrate was poured into 50 mL of ice-pure water, stirred, and after the substance was completely precipitated, it was filtered to obtain 2.9 g of the precipitate (compound 4), with a yield of 68.4%.
Embodiment 2
[0012] Embodiment 2: the synthesis of 2-methylamino-5-nitroaniline (compound 4)
[0013] 5.0 g of 2,4-dinitro-N-methylaniline (compound 3) was dissolved in 30 mL of acetonitrile, and 0.5 g of palladium carbon (water content 48%) was added, then the temperature was lowered to -20°C, and formic acid was added dropwise to the acetonitrile solution (5mL of formic acid was added to 5mL of acetonitrile to configure). After the dropwise addition was completed, the temperature was raised to reflux for 3 hours, and the filtrate was collected by filtration. The filtrate was poured into 50 mL of ice-cold pure water, stirred, and filtered to obtain 30.5 g of the precipitate (compound 4), with a yield of 71.9%.
Embodiment 3
[0014] Embodiment 3: the synthesis of 2-methylamino-5-nitroaniline (compound 4)
[0015] 5.0 g of 2,4-dinitro-N-methylaniline (compound 3) was dissolved in 25 mL of acetonitrile, and 0.375 g of palladium carbon (water content 48%) was added, then the temperature was lowered to -30°C, and formic acid was added dropwise to the acetonitrile solution (5mL of formic acid was added to 5mL of acetonitrile to configure). After the dropwise addition was completed, the temperature was raised to reflux for 3 hours, and the filtrate was collected by filtration. The filtrate was poured into 62.5 mL of ice-cold pure water, stirred, and filtered to obtain 123.2 g of the precipitate (compound 4), with a yield of 72.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com