Method for preparing bendamustine hydrochloride freeze-dried powder injection
A technology of bendamustine hydrochloride, freeze-dried powder injection, applied in the field of preparation of bendamustine hydrochloride freeze-dried powder injection, can solve the problems of high toxicity, increased production cost, poor safety, etc., and reach the content of related substances Low cost, save testing cost, reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
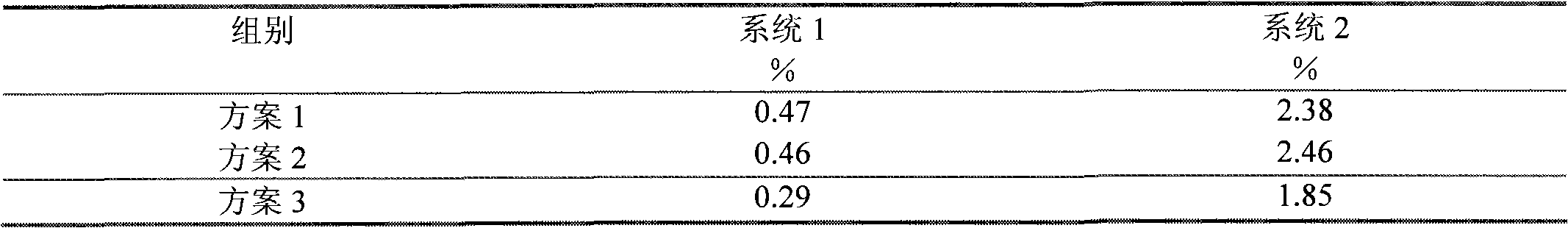
Embodiment 1
[0063] Add bendamustine hydrochloride and lyophilized excipients into water for injection at 30°C, dissolve, quickly add water for injection at 10°C, mix well, turn on the cooling system, cool the liquid rapidly at 10°C, adjust the pH value, pH When the value is 2.0, add activated carbon, stir, filter out the activated carbon, filter, fill, freeze-dry, and obtain.
Embodiment 2
[0065] Add bendamustine hydrochloride and lyophilized excipients into water for injection at 50°C, dissolve, quickly add water for injection at 0°C, mix well, turn on the cooling system, quickly cool the liquid to 0°C, and adjust the pH value, pH When the value is 2.0, add activated carbon, stir, filter out the activated carbon, filter, fill, freeze-dry, and obtain.
Embodiment 3
[0067] Add bendamustine hydrochloride and freeze-dried excipients into water for injection at 30°C, dissolve, quickly add water for injection at 0°C, mix well, turn on the cooling system, quickly cool the liquid to 0°C, and adjust the pH value, pH When the value is 2.0, add activated carbon, stir, filter out the activated carbon, filter, fill, freeze-dry, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com