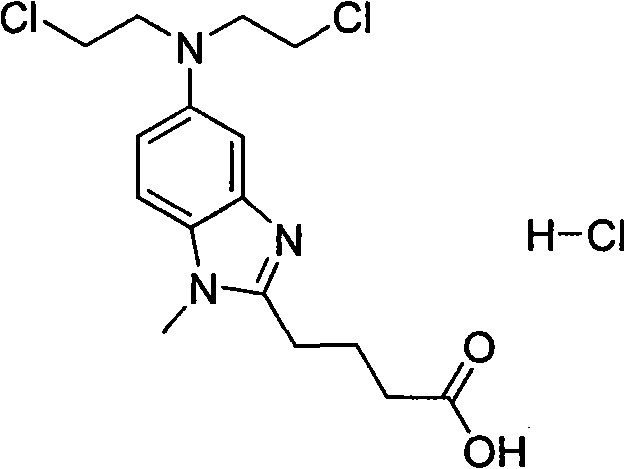
Bendamustine hydrochloride freeze-dried powder injection for injection and preparation method thereof
A technology of bendamustine hydrochloride and freeze-dried powder injection, which is applied in the field of freeze-dried powder injection of bendamustine hydrochloride for injection and its preparation, and can solve the problems of difficult redissolution, rough powder cake, and easily broken powder, etc. problem, to achieve the effect of no organic solvent residue, simple production process and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Bendamustine Hydrochloride Freeze-Dried Powder for Injection
[0025] Weigh 2.5 grams of mannitol, stir and dissolve in 80 ml of water for injection at 5°C; add 1.5 grams of bendamustine hydrochloride, stir and dissolve; dilute to 100 ml with water for injection; filter with a 0.22 μm microporous membrane, The filtrate is filtered through a 10,000-molecular-weight ultrafiltration membrane to remove pyrogens, then packaged, freeze-dried, and sealed to obtain bendamustine hydrochloride freeze-dried powder for injection.
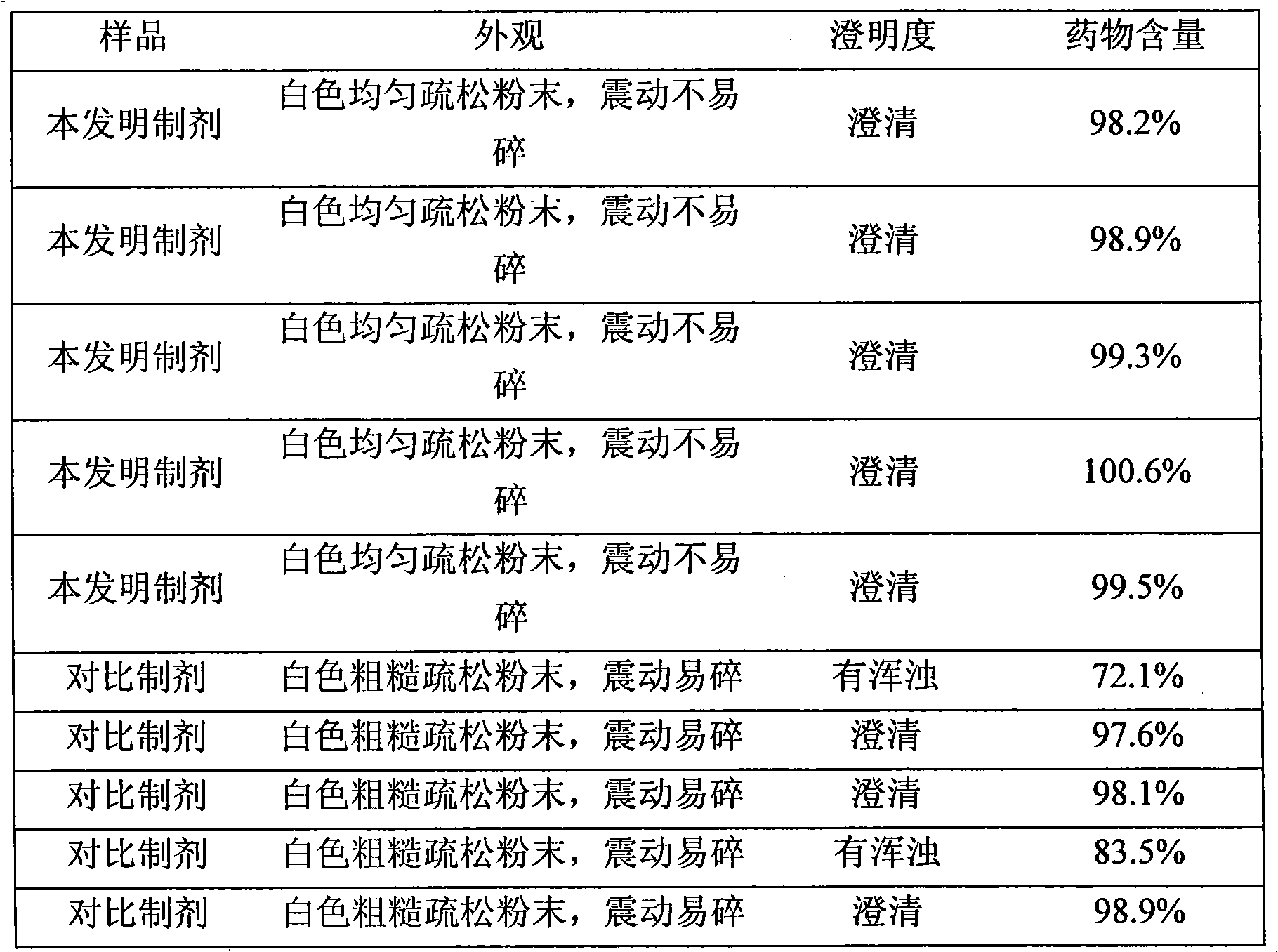
[0026] Through measurement, after the freeze-dried powder injection is reconstituted with water for injection, the solution is clear; the marked content is 100.2%.
Embodiment 2
[0027] Example 2 Bendamustine Hydrochloride Freeze-dried Powder for Injection
[0028] Weigh 3 grams of mannitol, stir and dissolve in 80 ml of water for injection at 7°C; add 2 grams of bendamustine hydrochloride, stir and dissolve; dilute to 100 ml with water for injection; filter with a 0.45 μm microporous membrane, The filtrate is filtered through a 5000 molecular weight ultrafiltration membrane to remove pyrogens, then packaged, freeze-dried, and sealed to obtain bendamustine hydrochloride freeze-dried powder for injection.
[0029] Through measurement, after the freeze-dried powder injection is reconstituted with water for injection, the solution is clear; the marked content is 99.4%.
Embodiment 3
[0030] Example 3 Bendamustine Hydrochloride Freeze-Dried Powder for Injection
[0031] Weigh 4 grams of mannitol, stir and dissolve in 80 ml of water for injection at 3°C; add 1 g of bendamustine hydrochloride, stir and dissolve; dilute to 100 ml with water for injection; filter with a 0.45 μm microporous membrane, The filtrate is filtered through a 10,000-molecular-weight ultrafiltration membrane to remove pyrogens, then packaged, freeze-dried, and sealed to obtain bendamustine hydrochloride freeze-dried powder for injection.
[0032] Through measurement, after the freeze-dried powder injection is reconstituted with water for injection, the solution is clear; the marked content is 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com