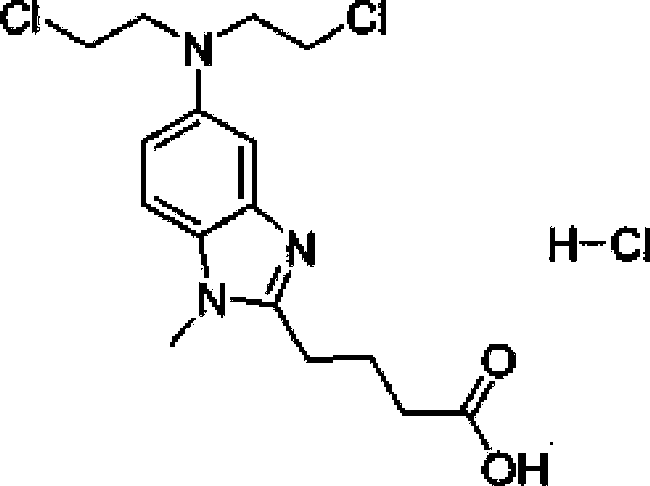
Bendamustine hydrochloride freeze-dried powder injection preparation method, product prepared through method, and use of product
A technology of bendamustine hydrochloride and freeze-dried powder injection, which is applied in the field of preparation of bendamustine hydrochloride preparations, can solve problems such as easy hydrolysis, and achieve the effects of loose and plump appearance, increased content and improved drying efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example is used to illustrate the preparation of bendamustine hydrochloride freeze-dried powder injection of the present invention.
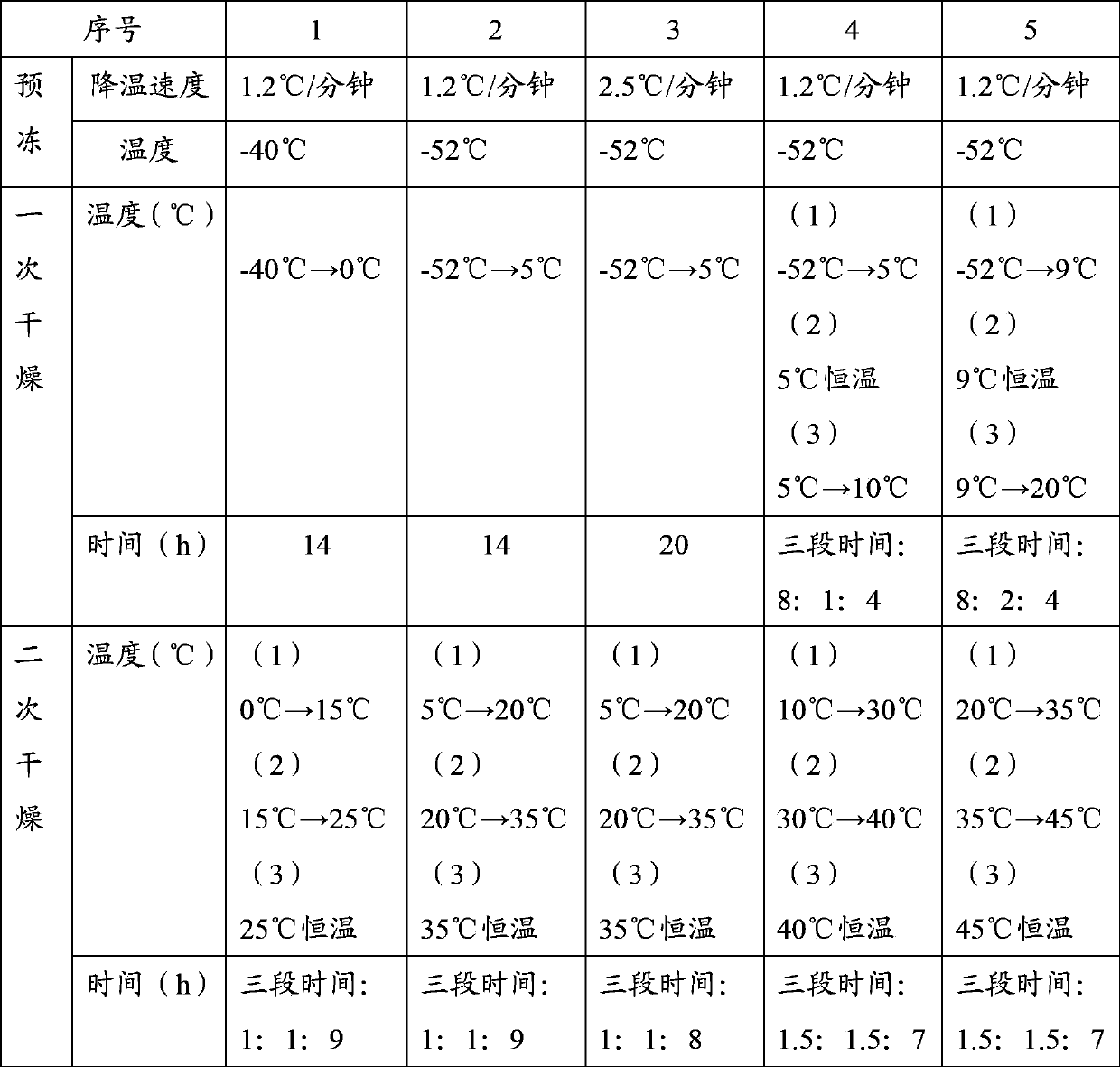
[0041] Pre-cool the brown neutral borosilicate glass injection bottle in cold hydrazine. Pass the bendamustine hydrochloride raw material through a 150-mesh sieve for later use. Accurately weigh 25g of bendamustine hydrochloride and 30g of mannitol, add them into the liquid mixing tank, add 4500ml of water for injection cooled to 4~8°C, stir rapidly to dissolve, then add the remaining amount of water for injection to make up to 5000ml, Store in an ice bath (4-8°C).
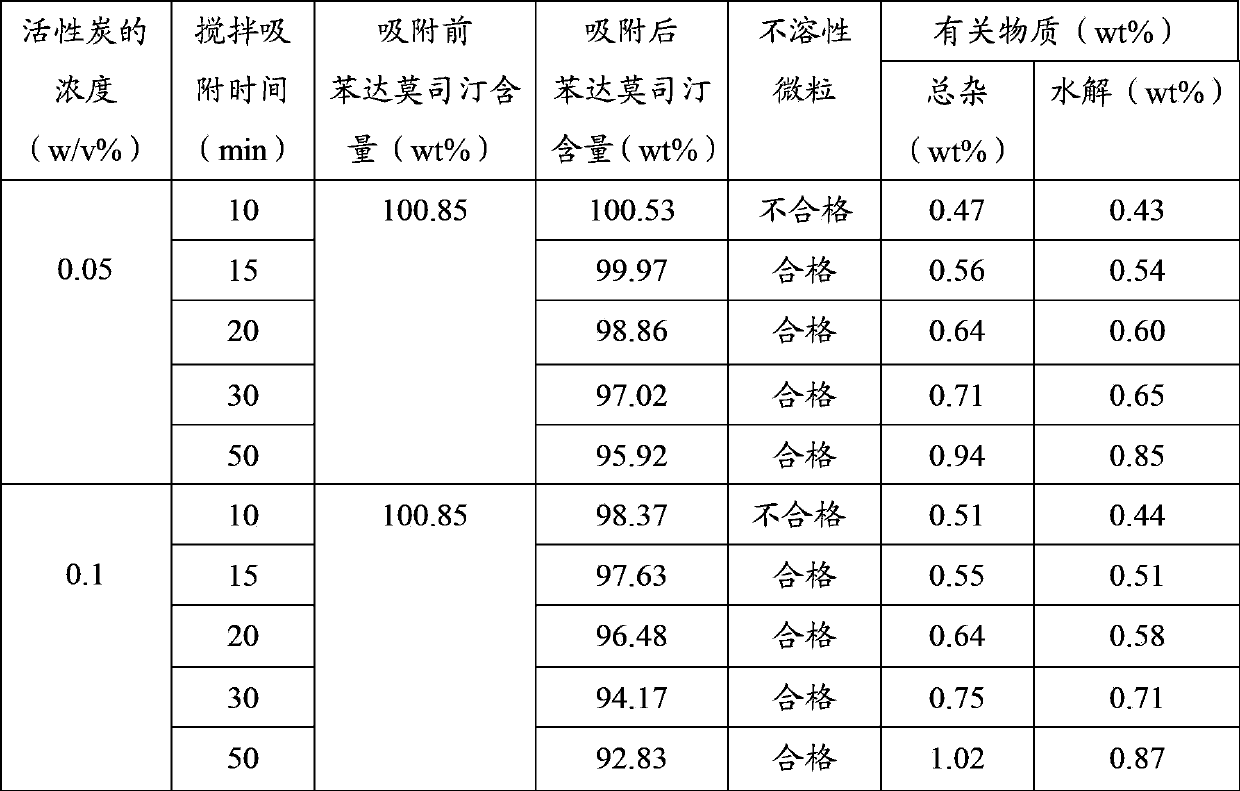
[0042] Add 2.5 g of activated carbon to the resulting mixed solution, stir with an automatic stirrer for 15 minutes under ice bath (4-8 ° C), filter and decarburize, and use a 0.22 μm sterilizing microporous filter membrane to fine-filter the filtrate to obtain the drug liquid, and then measure the content of the liquid medicine, and pack it into 1000 injection bottles...
Embodiment 2
[0045] This example is used to illustrate the preparation of bendamustine hydrochloride freeze-dried powder injection of the present invention.
[0046] Pre-cool the brown neutral borosilicate glass injection bottle in cold hydrazine. Pass the bendamustine hydrochloride raw material through a 120-mesh sieve for later use. Accurately weigh 25g of bendamustine hydrochloride and 35g of mannitol, put them into the liquid mixing tank, add 4500ml of water for injection cooled to 4~8°C, stir quickly to dissolve, then add the remaining amount of water for injection to dilute to 5000ml, Store in an ice bath (0-4°C).
[0047] Add 2.5g of activated carbon to the resulting mixed solution, stir with an automatic stirrer for 15 minutes under ice bath (0-4°C), filter and decarburize, and use a 0.22 μm sterilizing microporous filter membrane to fine-filter the filtrate to obtain the drug liquid, and then measure the content of the liquid medicine, and pack it into 1000 injection bottles aft...
Embodiment 3
[0050] This example is used to illustrate the preparation of bendamustine hydrochloride freeze-dried powder injection of the present invention.
[0051] Pre-cool the brown neutral borosilicate glass injection bottle in cold hydrazine. Pass the bendamustine hydrochloride raw material through a 100-mesh sieve for later use. Accurately weigh 25g of bendamustine hydrochloride and 40g of mannitol, add them into the liquid mixing tank, add 2700ml of water for injection cooled to 4~8°C, stir rapidly to dissolve, then add the remaining amount of water for injection to dilute to 3000ml, Store in an ice bath (4-8°C).
[0052] Add 1.2g of activated carbon to the resulting mixed solution, stir with an automatic stirrer for 10 minutes under ice bath (4~8°C) to filter and decarburize, and use a 0.22μm sterile microporous filter membrane to fine filter the filtrate to obtain a medicinal solution , and then measure the content of the liquid medicine, and pack it into 1000 injection bottles ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com