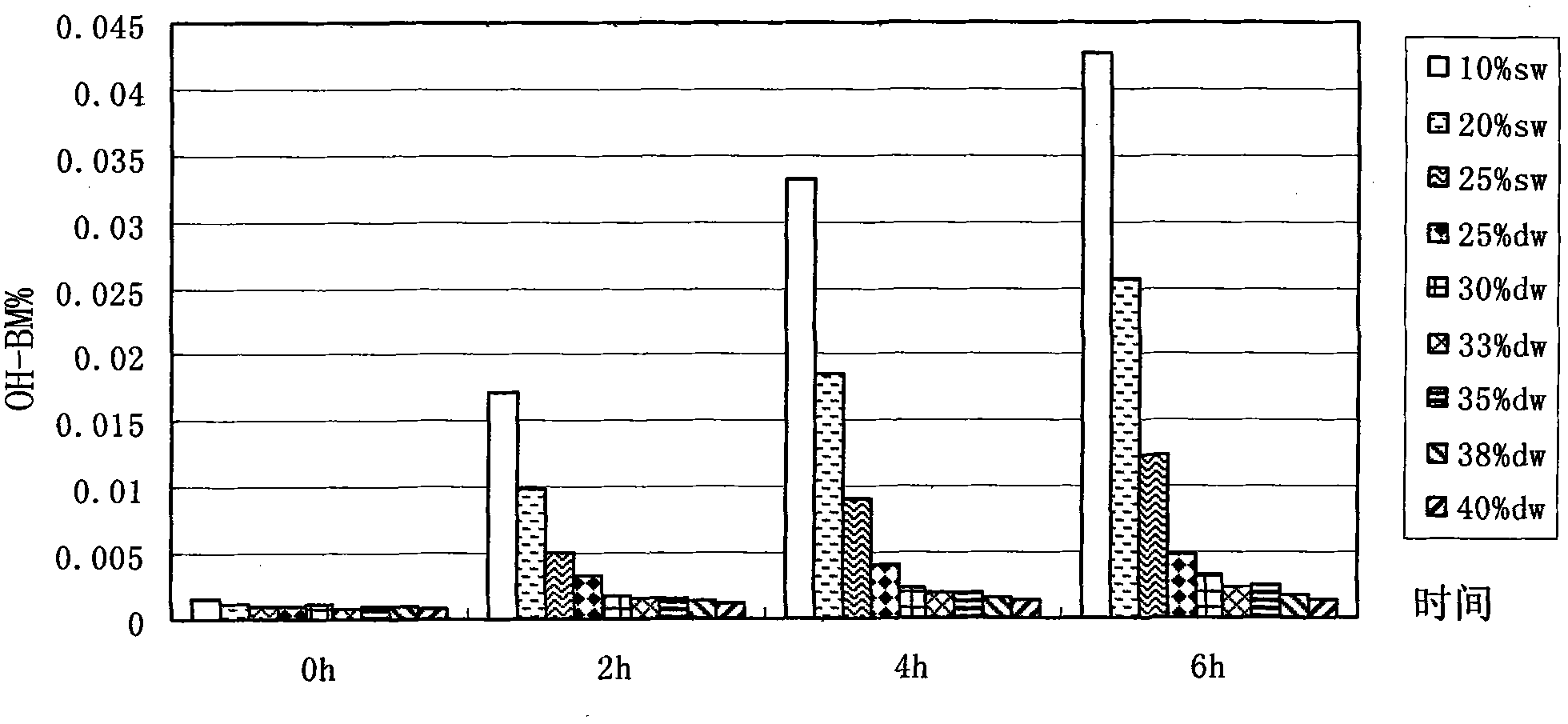
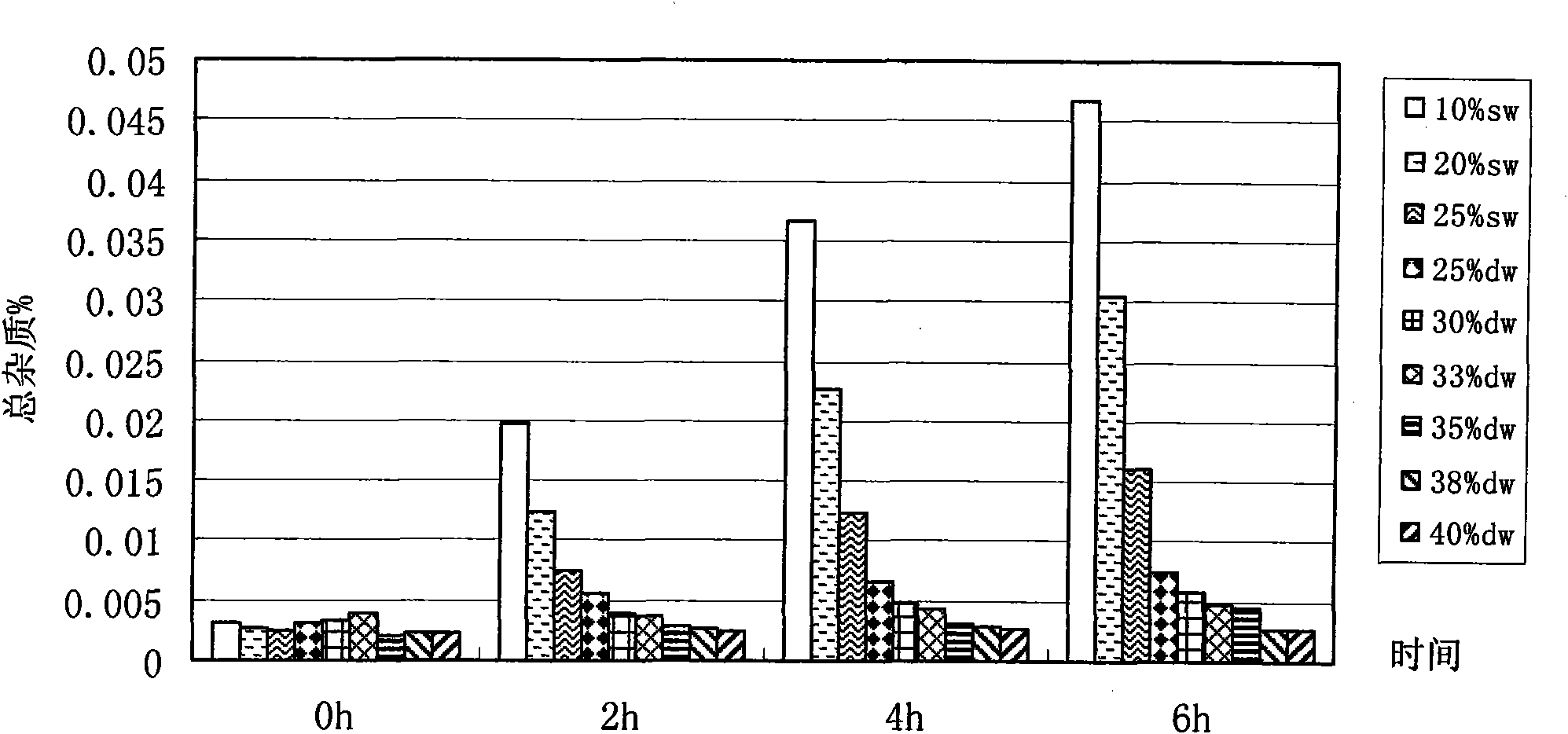
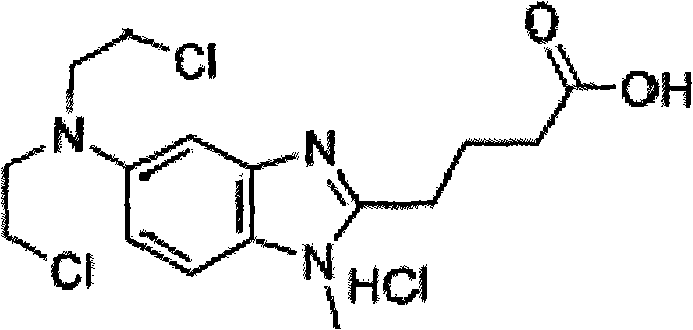
Method for preparing bendamustine hydrochloride freeze-dried preparation
A technology of bendamustine hydrochloride and freeze-dried preparations, which is applied in the field of preparation of pharmaceutical compositions, can solve problems such as difficult cakes, potential safety hazards, and difficulties in reconstitution, and achieve low organic solvent residues and slow down hydrolysis The effect of speed and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] Preparation Process:
[0039] Quickly dissolve bendastine hydrochloride in 0.45L of medicinal ethanol, then add it into 1.05L of water for injection in which 42.5g of mannitol has been dissolved, mix well, store at 5°C, filter and sterilize, sub-package, and send to freezer drying machine, freeze-drying to remove ethanol and water, and obtain a freeze-dried preparation of bendamustine hydrochloride.
[0040]The freeze-drying process includes the following steps: sending the subpackaged pre-freeze-drying solution into the freeze-drying box, turning on the freeze-drying machine circulation pump, compressor, and plate cooling valve, and quickly freeze-drying to -45°C, and keeping it for 5 Hours; open the condensation valve and vacuum system, control the vacuum at 20Pa, slowly enter the first drying stage, control the temperature of the freeze-dried sample at -20°C, and dry for 12 hours; quickly enter the second drying stage, and control the temperature of the f...
Embodiment 2
[0042]
[0043] Preparation Process:
[0044] Quickly dissolve bendastine hydrochloride in 0.375L of medicinal ethanol, then add it into 1.125L of water for injection in which 50g of lactose has been dissolved, mix well, store at 8°C, filter and sterilize, sub-package, and send it to a freeze dryer , and freeze-drying to remove ethanol and water to prepare a freeze-dried preparation of bendamustine hydrochloride.
[0045] The freeze-drying process includes the following steps: sending the subpackaged pre-freeze-drying solution into the freeze-drying box, turning on the freeze-drying machine circulation pump, compressor, and plate cooling valve, and quickly freeze-drying to -40°C, keeping the temperature for 6 Hours; open the condensation valve and vacuum system, control the vacuum degree at 20Pa, slowly enter the first drying stage, control the temperature of the freeze-dried sample at -30°C, and dry for 10 hours; quickly enter the second drying stage, and control the tempe...
Embodiment 3
[0047]
[0048] Preparation Process:
[0049] Quickly dissolve bendustine hydrochloride in 0.525L of medicinal ethanol, then add it into 0.975L of water for injection in which 37.5g of mannitol has been dissolved, mix well, store at 3°C, filter and sterilize, subpackage, and send to freezer drying machine, freeze-drying to remove ethanol and water, and obtain a freeze-dried preparation of bendamustine hydrochloride.
[0050] The freeze-drying process includes the following steps: sending the subpackaged pre-freeze-drying solution into the freeze-drying box, turning on the freeze-drying machine circulation pump, compressor, and plate cold valve, and rapidly freeze-drying to -50°C, and keeping it for 4 Hours; open the condensation valve and vacuum system, control the vacuum at 30Pa, slowly enter the first drying stage, control the temperature of the freeze-dried sample at -25°C, and dry for 11 hours; quickly enter the second drying stage, and control the temperature of the fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com