A Stannic Oxide Microballoon with Controllable Crystallite Dimension and its Preparation Method and Application
A technology of tin dioxide and microcrystalline size, which is applied in tin oxide, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., and can solve the problems of small grains that are easy to agglomerate, unfavorable ion penetration, and unfavorable sunlight. Light harvesting and other issues can be achieved to promote nucleation and growth, the process route is simple, and the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Measure 50 milliliters of n-propanol, add 1.5 milliliters of concentrated hydrochloric acid and 0.4513 gram of tin protochloride dihydrate successively under magnetic stirring condition, form transparent homogeneous solution; The filling rate of the reaction kettle is 60%, the reaction temperature is controlled at 200° C., and the reaction time is 12 hours; after the reaction is completed, the precipitate is taken out, washed repeatedly with pure water, and centrifuged.
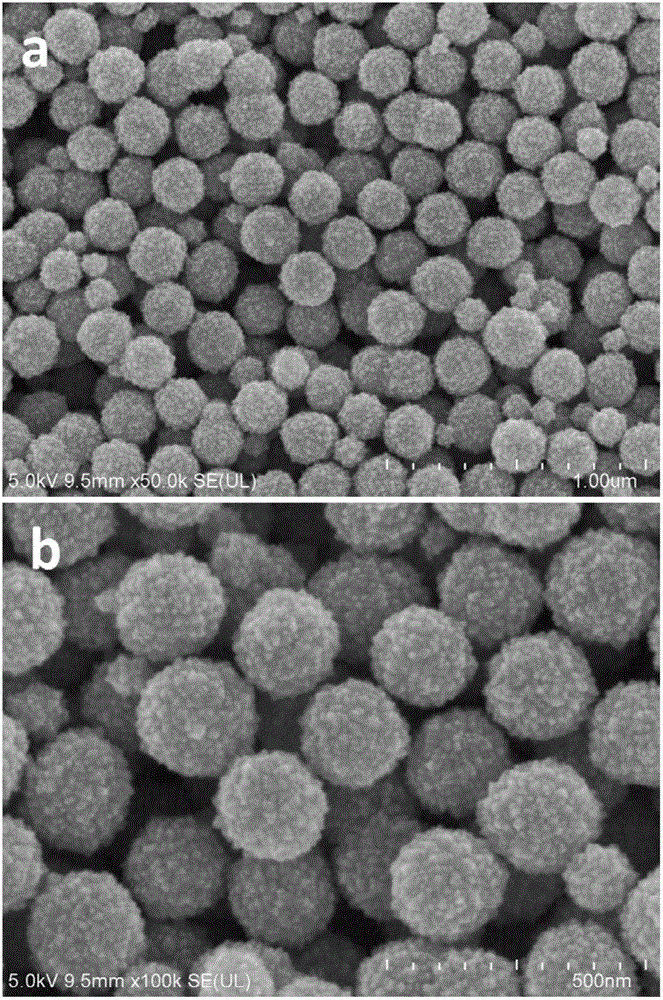
[0041] figure 1 For the field emission scanning electron micrograph of the tin dioxide microspheres prepared in Example 1, it can be seen from the figure that the dispersion of the microspheres is good, the size distribution is uniform, and the diameter of a single microsphere is 200 to 300 nanometers; It can also be seen from the scanning electron microscope that the microspheres are formed by the aggregation of small grains, and the grain size of the microcrystals is about 10 nanometers.
Embodiment 2
[0043] Mix 2.5 milliliters of pure water and 47.5 milliliters of n-propanol, add 1.5 milliliters of concentrated hydrochloric acid and 0.4513 grams of stannous chloride dihydrate in sequence under magnetic stirring conditions to form a transparent homogeneous solution; transfer the above-mentioned transparent solution to the reaction kettle Carry out solvothermal reaction, the filling rate of the reactor is 60%, the reaction temperature is controlled at 200° C., and the reaction time is 12 hours; after the reaction is completed, the precipitate is taken out, repeatedly washed with pure water, and centrifuged.
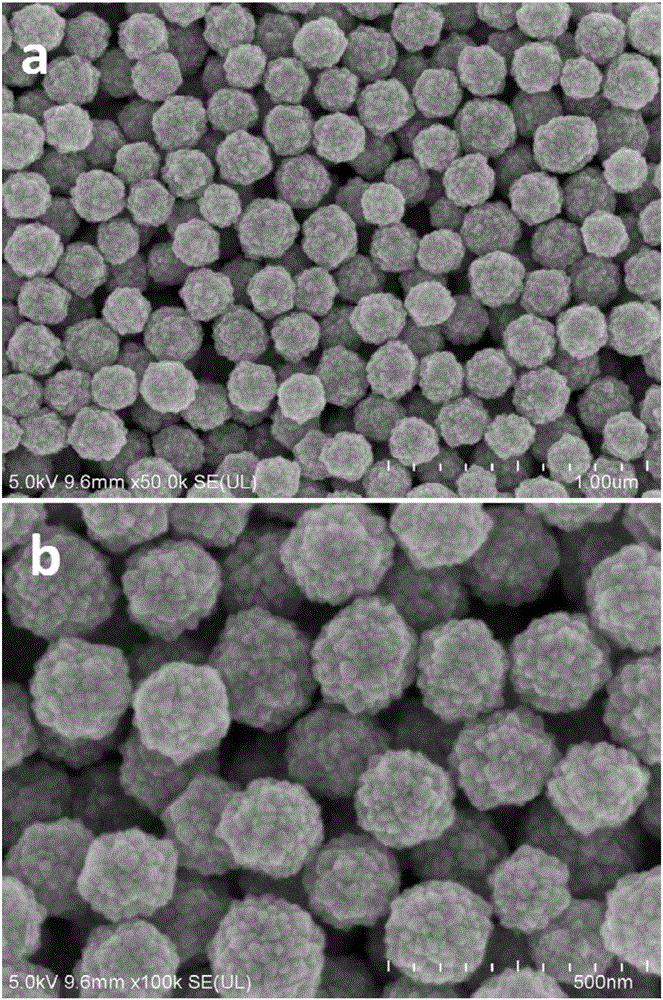
[0044] figure 2 For the field emission scanning electron micrograph of the tin dioxide microspheres prepared in Example 2, it can be seen from the figure that the dispersion of the microspheres is good, the size distribution is uniform, and the diameter of a single microsphere is 200 to 300 nanometers; It can also be seen from the scanning electron microscope that the ...
Embodiment 3
[0046] Mix 5 milliliters of pure water and 45 milliliters of n-propanol, add 1.5 milliliters of concentrated hydrochloric acid and 0.4513 grams of stannous chloride dihydrate successively under the condition of magnetic stirring to form a transparent homogeneous solution; transfer the above-mentioned transparent solution to the reaction kettle Carry out solvothermal reaction, the filling rate of the reactor is 60%, the reaction temperature is controlled at 200° C., and the reaction time is 12 hours; after the reaction is completed, the precipitate is taken out, repeatedly washed with pure water, and centrifuged.
[0047] image 3 The field emission scanning electron microscope image of the tin dioxide microspheres prepared for Example 3 shows that the dispersion of the microspheres is good and the size is very uniform, and the diameter of a single microsphere is 250 nanometers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Grain size | aaaaa | aaaaa |
| Grain size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com