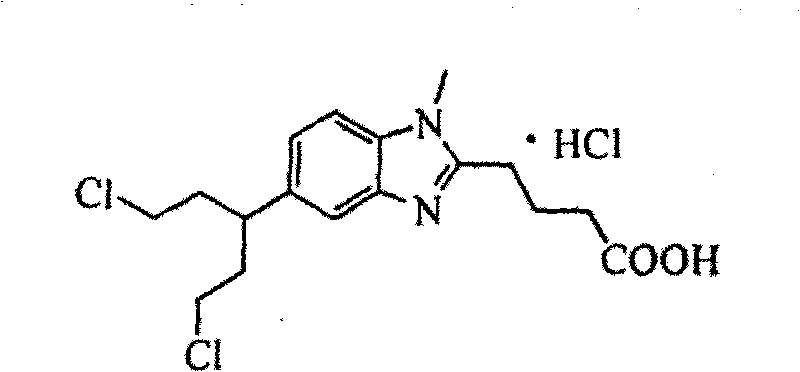
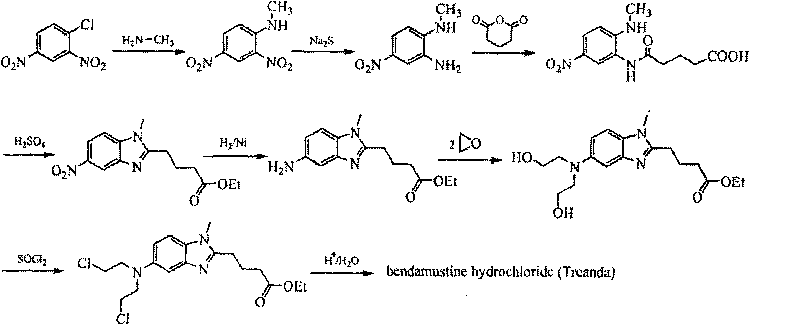
Method for synthesizing highly-pure bendamustine hydrochloride
A technology for the synthesis of bendamustine hydrochloride and a synthesis method, which is applied in the field of synthesis of bendamustine hydrochloride, can solve the problems of many impurities, difficult purification, and inability to synthesize, and achieve short synthesis period, easy separation, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) Synthesis of [1-methyl-2(4'-butyric acid ethyl)-5-N,N-bis(2'-hydroxyethyl)]-1H-benzimidazole
[0019] Stir and dissolve [1-methyl-2(4'-butanoic acid ethyl)-5-amino]-1H-benzimidazole (150g, 0.58mol), water 1500mL, and glacial acetic acid 750mL in a 3L reaction flask, Cool to -5~0°C, add 300mL ethylene oxide, and control the temperature until the reaction is complete. Adjust the pH to 7.1-7.3 with saturated potassium carbonate solution, extract with 800 mL×3 dichloromethane, combine the organic phases, wash with saturated brine 600 mL×3, and dry over anhydrous magnesium sulfate. Suction filtration and concentration gave a brown oily solid.
[0020] 2) Chlorination reaction to synthesize [1-methyl-2(4'-butyric acid ethyl)-5-N,N-bis(2'-chloroethyl)]-1H-benzimidazole
[0021] [1-Methyl-2(4'-butanoic acid ethyl)-5-N,N-bis(2'-hydroxyethyl)]-1H-benzimidazole (200g, 0.56mol), xylene 800mL, phosphorus oxychloride 500mL mixed, heated to reflux for 8h. Allow to cool, and con...
Embodiment 2
[0029] 1) Synthesis of [1-methyl-2(4'-butyric acid ethyl)-5-N,N-bis(2'-hydroxyethyl)]-1H-benzimidazole
[0030] Stir and dissolve [1-methyl-2(4'-butanoic acid ethyl)-5-amino]-1H-benzimidazole (150g, 0.58mol), water 1500mL, and glacial acetic acid 750mL in a 3L reaction flask, Cool to -5~0°C, add 250mL ethylene oxide, and control the temperature until the reaction is complete. Adjust the pH to 7.1-7.3 with saturated potassium carbonate solution, extract with 800 mL×3 dichloromethane, combine the organic phases, wash with saturated brine 600 mL×3, and dry over anhydrous magnesium sulfate. Suction filtration and concentration gave a brown oily solid.
[0031] 2) Chlorination reaction to synthesize [1-methyl-2(4'-butyric acid ethyl)-5-N,N-bis(2'-chloroethyl)]-1H-benzimidazole
[0032] [1-Methyl-2(4'-butanoic acid ethyl)-5-N,N-bis(2'-hydroxyethyl)]-1H-benzimidazole (200g, 0.56mol), toluene 1000mL , phosphorus oxychloride 800mL mixed, heated to reflux for 3h. Allow to cool, and ...
Embodiment 3
[0038] 1) Synthesis of [1-methyl-2(4'-butyric acid ethyl)-5-N,N-bis(2'-hydroxyethyl)]-1H-benzimidazole
[0039] Stir and dissolve [1-methyl-2(4'-butanoic acid ethyl)-5-amino]-1H-benzimidazole (150g, 0.58mol), water 1500mL, and glacial acetic acid 750mL in a 3L reaction flask, Cool to -5~0°C, add 400mL ethylene oxide, and control the temperature until the reaction is complete. Adjust the pH to 7.1-7.3 with saturated potassium carbonate solution, extract with 800 mL×3 dichloromethane, combine the organic phases, wash with saturated brine 600 mL×3, and dry over anhydrous magnesium sulfate. Suction filtration and concentration gave a brown oily solid.
[0040] 2) Chlorination reaction to synthesize [1-methyl-2(4'-butyric acid ethyl)-5-N,N-bis(2'-chloroethyl)]-1H-benzimidazole
[0041] [1-Methyl-2(4'-butanoic acid ethyl)-5-N,N-bis(2'-hydroxyethyl)]-1H-benzimidazole (200g, 0.56mol), xylene 800mL, phosphorus oxychloride 1000mL mixed, heated to reflux for 5h. Allow to cool, and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com