Novel solid forms of bendamustine hydrochloride
a technology of bendamustine and hydrochloride, which is applied in the field of solid forms of bendamustine hydrochloride, can solve problems such as difficulties in its preparation and administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
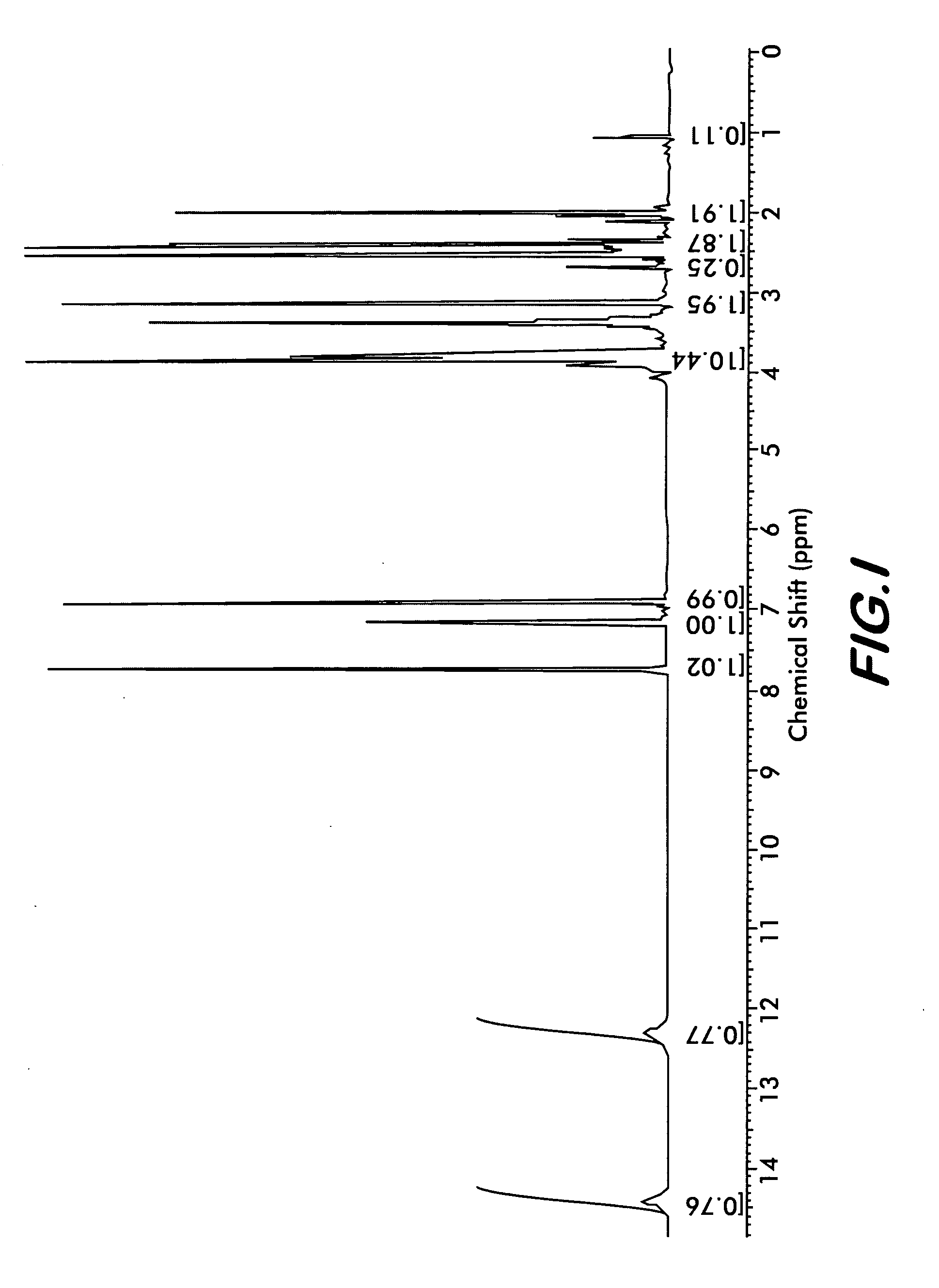
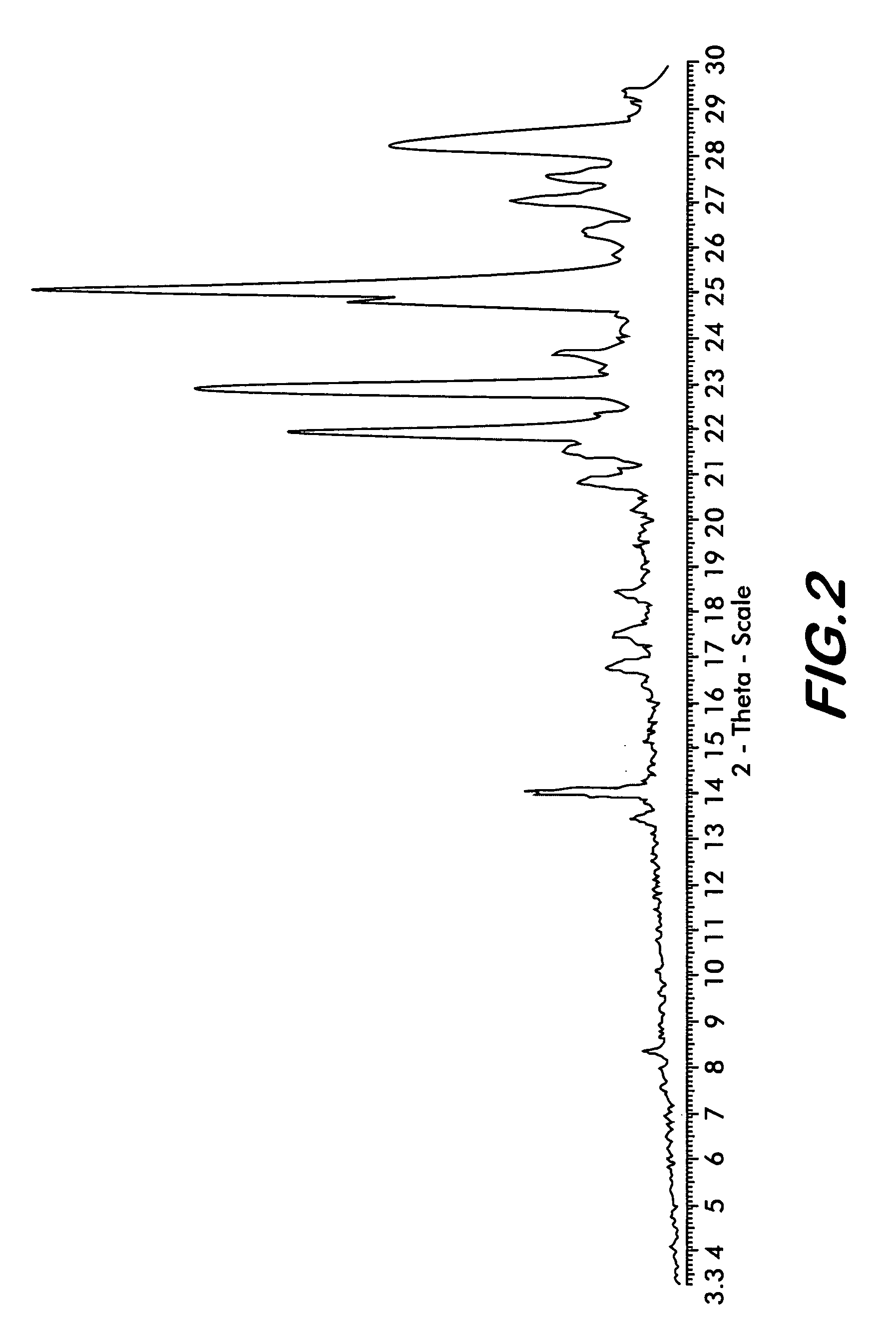
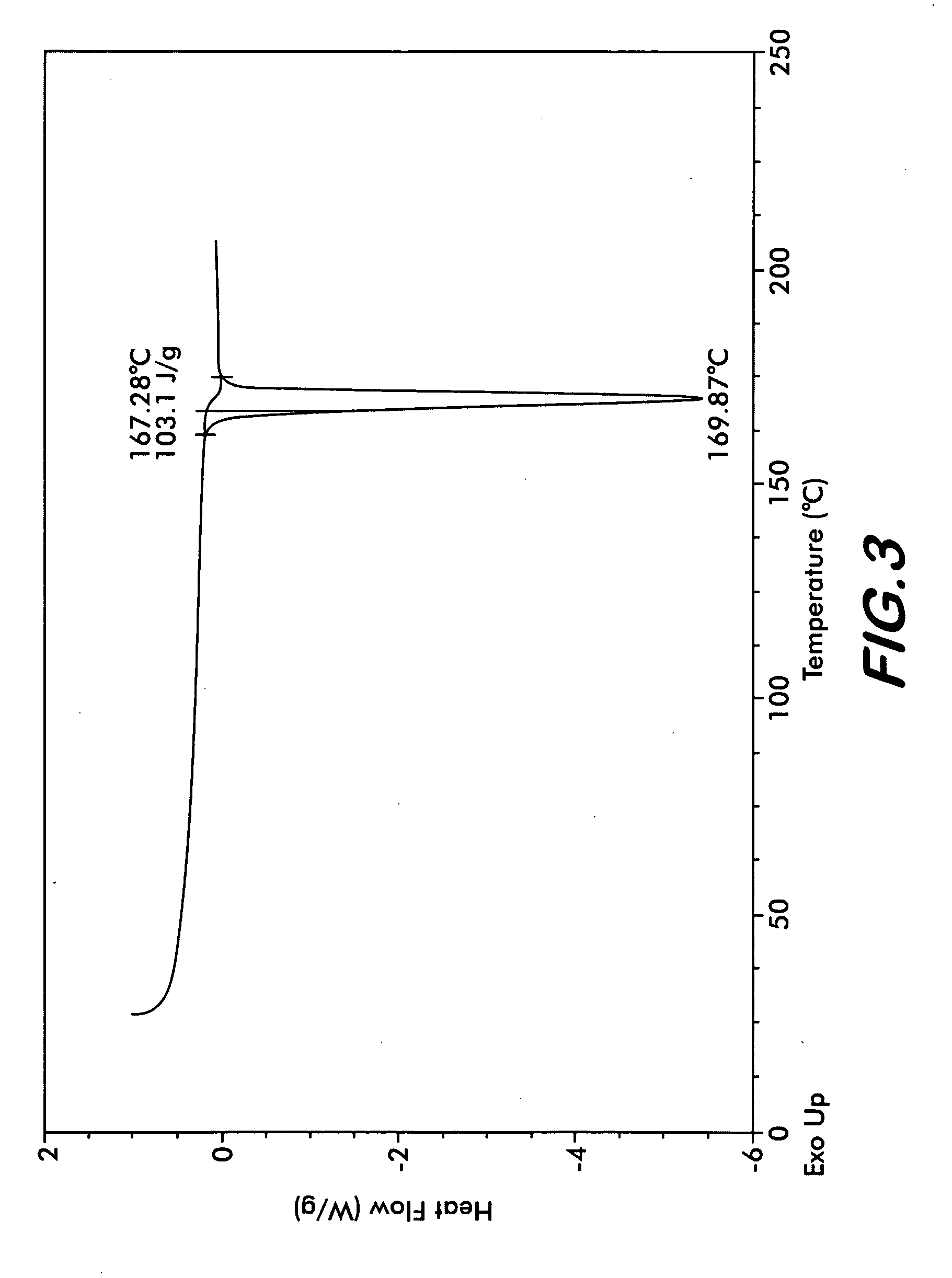
[0029]Four polymorphs of crystalline bendamustine hydrochloride are disclosed herein (referred to herein as Form 1, Form 2, Form 3, and Form 4). Also described is amorphous (i.e., non-crystalline) bendamustine hydrochloride. Spectral data relating to these solid forms of bendamustine hydrochloride is depicted in FIGS. 1-14, and methods of preparing each of these forms is presented
[0030]In preferred embodiments are solid forms of bendamustine hydrochloride that comprise Form 1, Form 2, Form 3, Form 4, or mixtures thereof. More preferred embodiments are solid forms of bendamustine hydrochloride that are Form 1, Form 3, Form 4, amorphous bendamustine hydrochloride, or mixtures thereof. In other embodiments, solid forms of the invention may further comprise bendamustine hydrochloride Form 2. These polymorphic solid forms may be identified, for example, by X-ray powder diffraction and characterized by one, two, three, four, five, or more reflection peaks that are characteristic of each p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| 2θ | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com