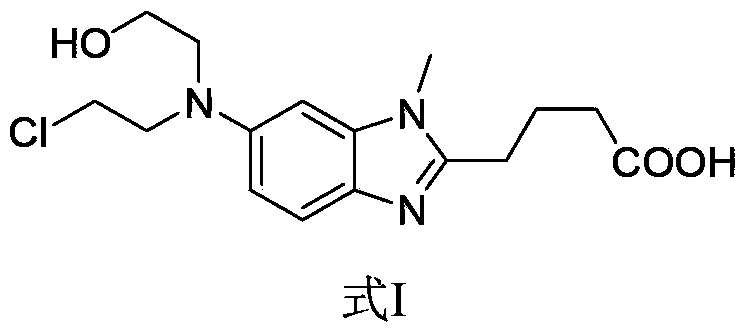
Preparation method of impurity HP1 in bendamustine hydrochloride
A technology of bendamustine hydrochloride and impurities, applied in the field of medicinal chemistry, to achieve the effect of high product purity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of bendamustine hydrochloride impurity HP1 comprises the following steps:
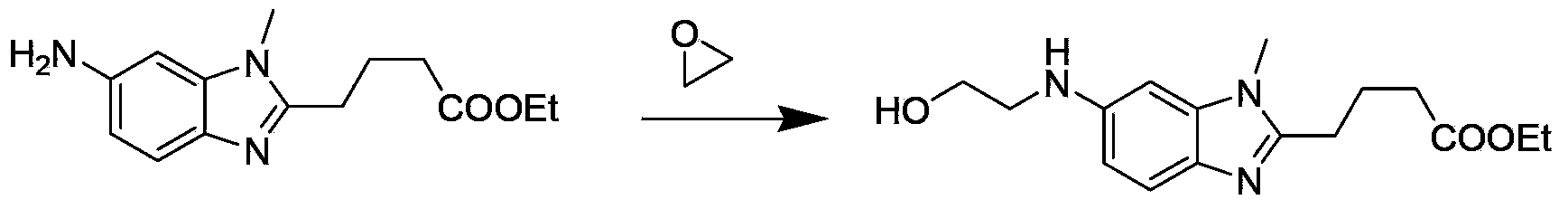
[0029] In the first step, [1-methyl-2-(4'-butyric acid ethyl)-5-amino]-1H-benzimidazole is used as the starting material, and the organic acid aqueous solution of C1~C4 is used as the solvent, React with ethylene oxide, adjust the pH after the reaction, extract the organic phase, wash, dry, and separate to obtain [1-methyl-2-(4'-butyric acid ethyl)-5-N-(2'-hydroxy Ethyl)]-1H-benzimidazole:
[0030]
[0031] Wherein, the molar ratio of [1-methyl-2-(4'-butanoic acid ethyl)-5-amino]-1H-benzimidazole to ethylene oxide is 1:1-2, preferably 1:1 ; The organic acid of C1~C4 is selected from one of formic acid, acetic acid, propionic acid, oxalic acid or n-butyric acid, preferably acetic acid; the solvent is preferably an aqueous solution of acetic acid with a volume ratio of 1:1; the reaction temperature is -10°C-5°C , preferably 0°C-5°C; the reaction time is 1h-6h.
[0032]...
Embodiment 1
[0041] Step 1: Preparation of [1-methyl-2-(4'-butyric acid ethyl)-5-N-(2'-hydroxyethyl)]-1H-benzimidazole
[0042] Add 10.0 g of [1-methyl-2-(4'-butanoic acid ethyl)-5-amino]-1H-benzimidazole, 50 mL of water, and 50 mL of acetic acid into a 200 mL single-necked bottle, and stir for 30 minutes, then add 4.0 mL of ethylene oxide, react in an ice-water bath for 4 hours, stop the reaction, adjust the pH to 7-8 with ammonia water, extract the reaction solution with dichloromethane (100 mL×2), and combine the organic phases. The organic phase was washed with saturated sodium chloride solution (80 mL×2), and dried over anhydrous sodium sulfate. Column chromatography (eluent: dichloromethane:methanol=100:2) separated to obtain 2.2g of the product (yellow oily liquid).
[0043] ESI-MS m / z:306.1[M+H + ] + .
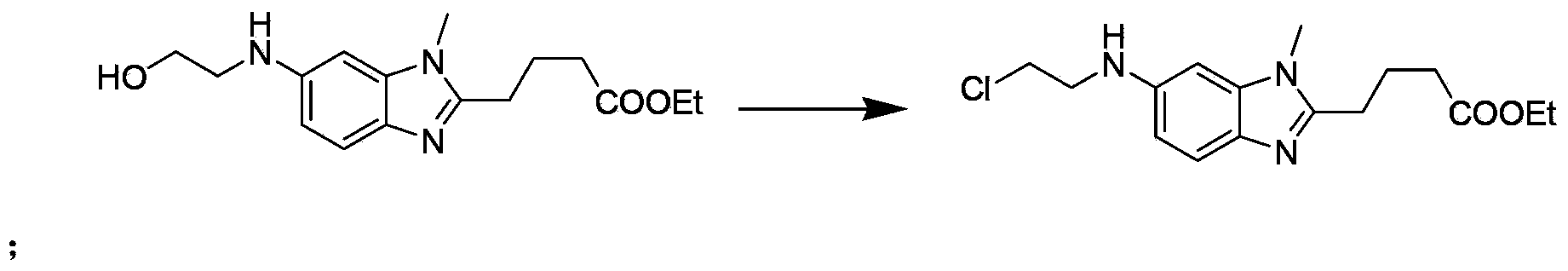
[0044] The second step: preparation of [1-methyl-2-(4'-butyric acid ethyl)-5-N-(2'-chloroethyl)]-1H-benzimidazole
[0045]Add 2.2 g of [1-methyl-2-(4'-butyric acid ethyl)-5-N-...
Embodiment 2
[0054] Step 1: Preparation of [1-methyl-2-(4'-butyric acid ethyl)-5-N-(2'-hydroxyethyl)]-1H-benzimidazole
[0055] Add 10.0 g of [1-methyl-2-(4'-butanoic acid ethyl)-5-amino]-1H-benzimidazole, 50 mL of water, and 50 mL of formic acid in sequence into a 200 mL single-necked bottle, and stir at -10°C After 30 minutes, add 4.0 mL of ethylene oxide, react at -10°C for 1 hour, stop the reaction, adjust the pH to 7-8 with ammonia water, extract the reaction solution with dichloromethane (100 mL×2), and combine the organic phases. The organic phase was washed with saturated sodium chloride solution (80 mL×2), and dried over anhydrous sodium sulfate. The product (yellow oily liquid) was separated by column chromatography (eluent: dichloromethane:methanol=100:2).
[0056] The second step: preparation of [1-methyl-2-(4'-butyric acid ethyl)-5-N-(2'-chloroethyl)]-1H-benzimidazole
[0057] Add 2.2 g of [1-methyl-2-(4'-butyric acid ethyl)-5-N-(2'-hydroxyethyl)]-1H-benzimidazole and 100 mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com