Method for preparing high-purity bendamustine hydrochloride
A technology for bendamustine hydrochloride and bendamustine hydrochloride crude product, which is applied in the field of preparation of high-purity bendamustine hydrochloride, can solve the problems of decreasing purity, failing to meet technical requirements, difficulties, etc. The effect of stability, improved product purity and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
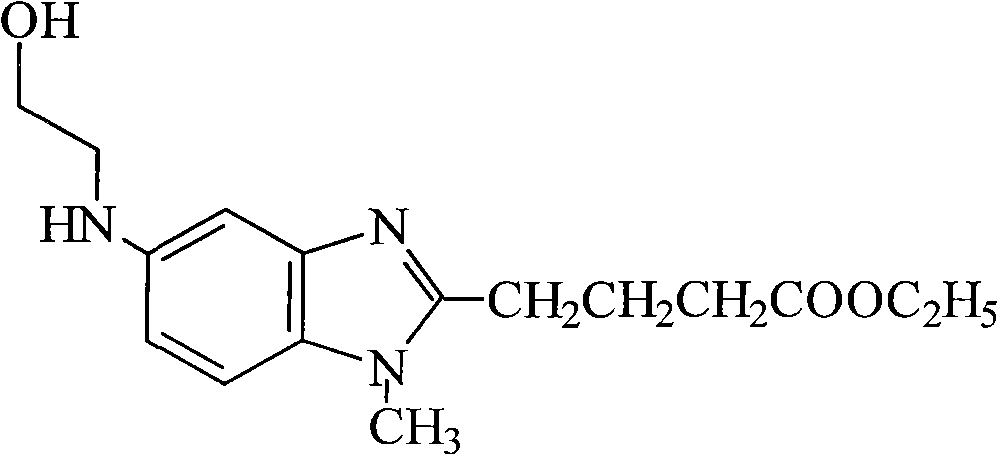
[0036] The preparation of the 4-[5-[bis-(2-hydroxyethyl) amino]-1-methyl-2-benzimidazole] ethyl butyrate (intermediate) of embodiment 1 oily or colloidal
[0037] Oily or gum-like ethyl 4-[5-[bis-(2-hydroxyethyl)amino]-1-methyl-2-benzimidazole]butanoate (intermediate) can refer to the literature Journal fur praktische Chemie. 4. Prepared by Reihe.Band20.1963 (178-186), it can also be prepared by referring to the following method:
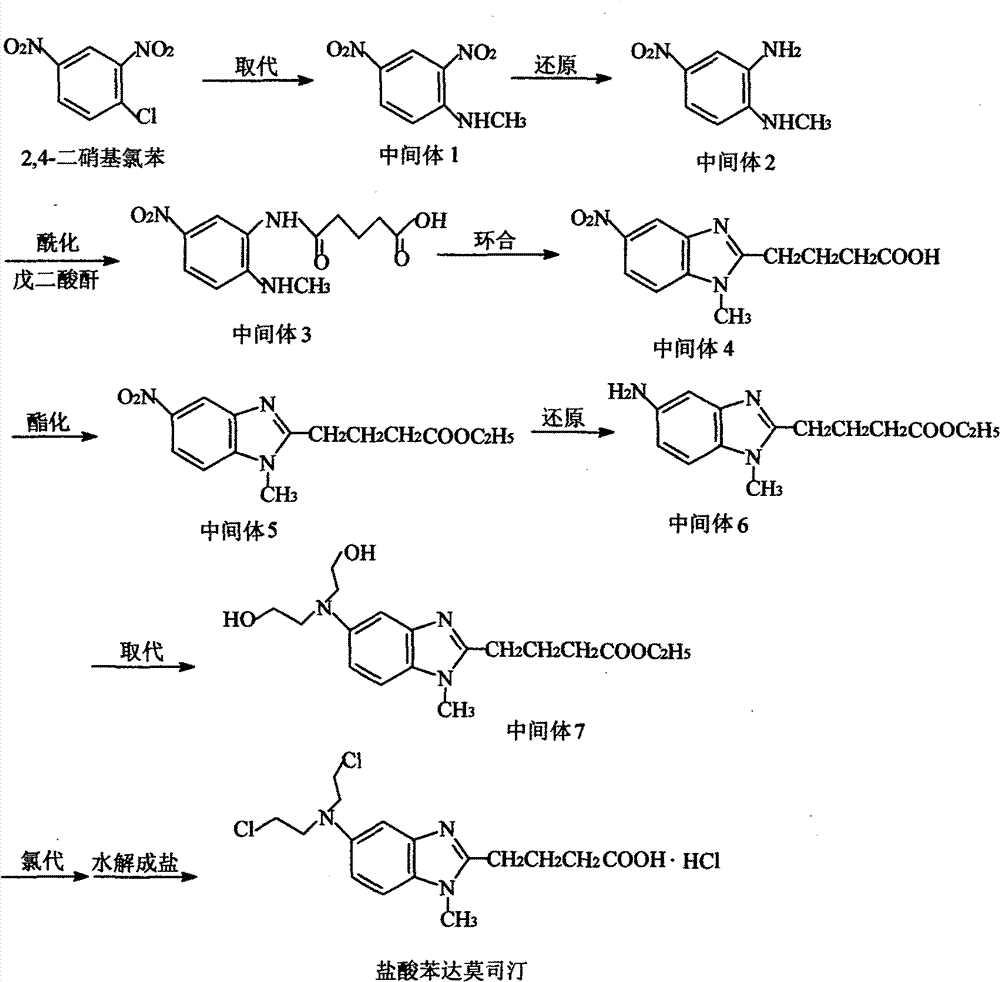
[0038] (1) Preparation of N-methyl-(2,4-dinitro)aniline (intermediate 1)
[0039] Add 438g of 2,4-dinitrochlorobenzene, 141g of methylamine hydrochloride, 573g of sodium acetate trihydrate and 1050ml of N,N-dimethylformamide into a 3L three-necked flask, heat to reflux, stir for 3h, TLC The reaction was stopped after detection of no raw material. After the reaction solution was slightly cooled, it was quickly poured into 3000ml of ice water, and allowed to stand for crystallization. After filtering, the filter cake was washed with an appropriate ...
Embodiment 2
[0061] Example 2 Preparation of 4-[5-[bis-(2-hydroxyethyl)amino]-1-methyl-2-benzimidazole] ethyl butyrate (intermediate) in solid form
[0062] Add ethyl acetate 100ml to the oily 4-[5-[bis-(2-hydroxyethyl)amino]-1-methyl-2-benzimidazole] ethyl butyrate 23g prepared in Example 1, and add ethyl acetate 100ml at room temperature (24°C) stirring and dissolving to obtain a clear solution, 1000ml of n-heptane was added dropwise to the solution to precipitate a solid, the mixture was stirred and crystallized at room temperature 7 (24°C) for about 30 minutes, the solid was separated by filtration, and air-dried at 40°C to obtain Off-white solid: 14.7g, yield: 64.1%;
[0063] mp: 105-106.5°C, HPLC purity 98.8%.
[0064] Elemental analysis (C 18 h 27 N 3 o 4 ): Theory: C 61.87%; H 7.79%; N 12.02%;
[0065] Measured: C 61.82%; H 7.82%; N 12.01%;
[0066]1 H-NMR (CD 3 OD):
[0067] δ6.59, 7.03, 7.54(m, Ar-H), δ4.12(m, 2H), δ3.76(m, 2H), δ3.62(s, 3H), δ3.54(m, 2H) , δ2.55(m, 2H)...
Embodiment 3
[0073] The preparation of embodiment 3 bendamustine hydrochloride
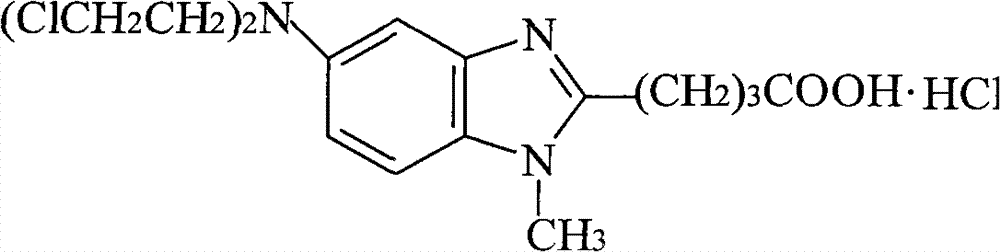
[0074] The solid intermediate 4-[5-[bis-(2-hydroxyethyl)amino]-1-methyl-2-benzimidazole] ethyl butyrate (17.5g, 0.05mol) prepared in Example 2 1. Add 350ml of dichloromethane into a 1L reaction flask, stir mechanically, cool to 0-5°C, add 30ml of thionyl chloride dropwise, keep it warm at 0-5°C for 1h, and then react at room temperature for 3h. Concentrate under reduced pressure to dryness, add 350ml of concentrated hydrochloric acid to the viscous liquid, heat to reflux for 3h, add 3g of activated carbon, stir for 10min, filter while hot, concentrate the filtrate to dryness, add the residue to 50ml of purified water, stir to precipitate a solid . After filtration, the solid was vacuum-dried at 50° C. for 5 hours to obtain 15.9 g of crude bendamustine hydrochloride, with a yield of 80.6%, a purity of 98.3%, and a maximum purity of 0.55%.
[0075] Put 15.9 g of the above bendamustine hydrochloride crude produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com