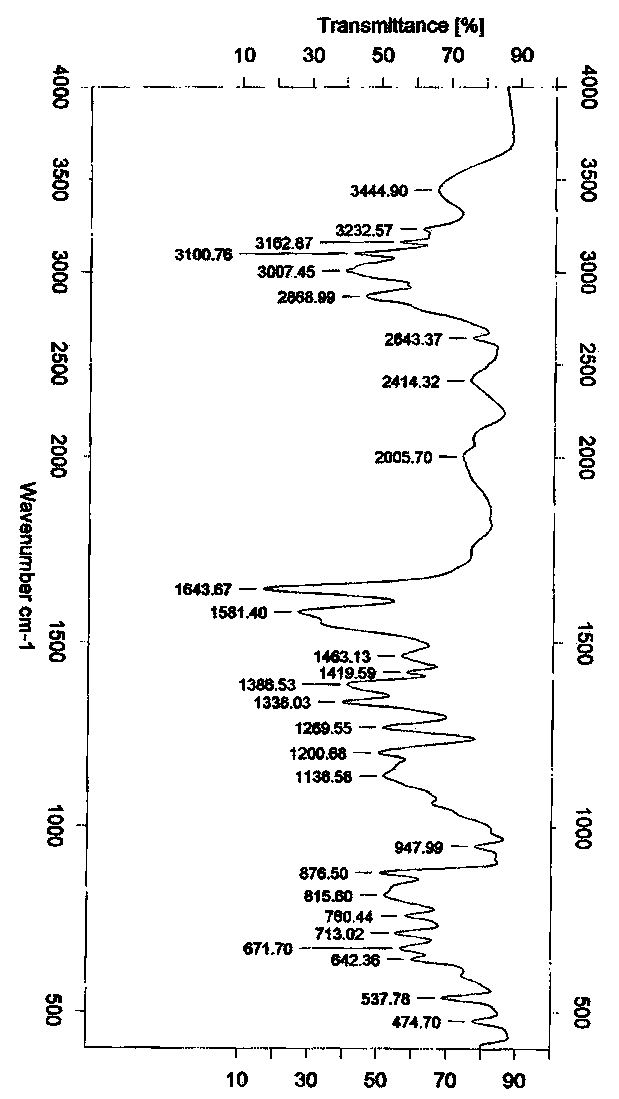
Ingavirin polymorph and its preparation method
A polymorph, ingavirine technology, which is applied in the field of polymorphs of ingavirine and its preparation, can solve the problem of affecting drug dissolution rate, affecting drug fluidity, tableting hardness, tablet weight difference and content uniformity Sexual and physical stability, affecting drug activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
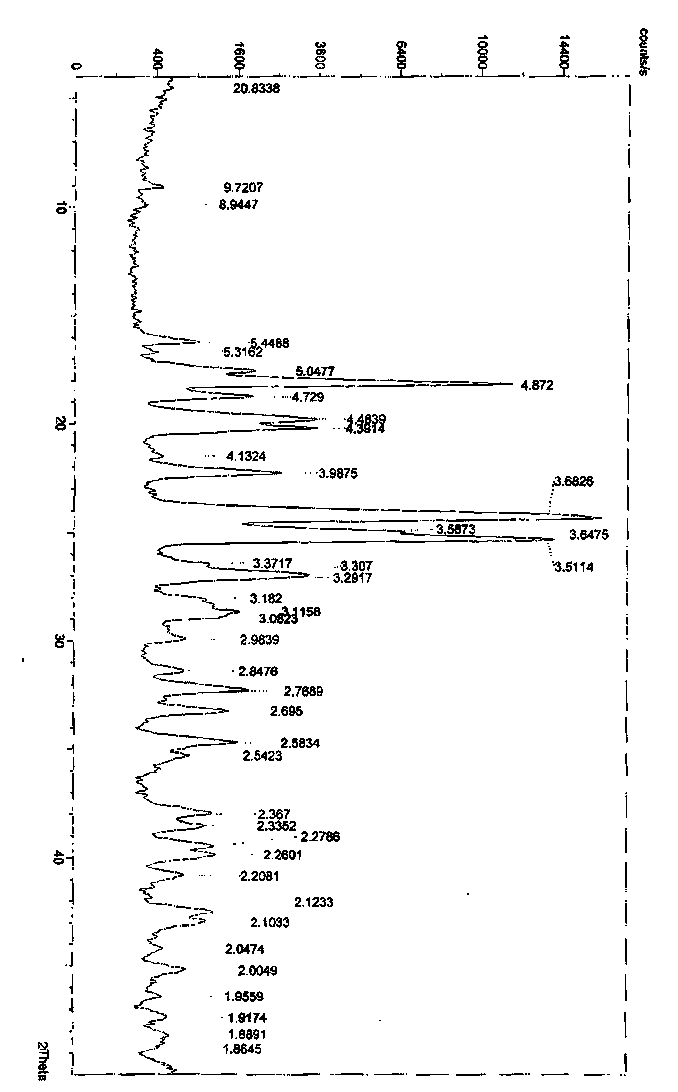
[0057] Embodiment 1: Preparation of ingaverine crystal form A
[0058] Dissolve 2 g of ingavirin solid in 14 mL of water, add 60 mL of acetone, a white solid precipitates out, stir in an ice bath for 2 h, filter, and wash the filter cake twice with acetone to obtain 1.4 g of ingavirin crystal form A. Yield: 70%.
Embodiment 2
[0059] Embodiment 2: Preparation of ingaverine crystal form A
[0060] Add 2.98g of crude ingavirin and 50mL of methanol into the reaction kettle, add 25mL of triethylamine dropwise with stirring until the solid dissolves, then slowly add 360mL of ethyl acetate, cool down to 0°C, stir and crystallize for 2h, filter, and filter the cake Wash with 100 mL of ethyl acetate, collect the filter cake, and dry under reduced pressure to obtain 1.94 g of white solid, which is Ingavirine Form A.
Embodiment 3
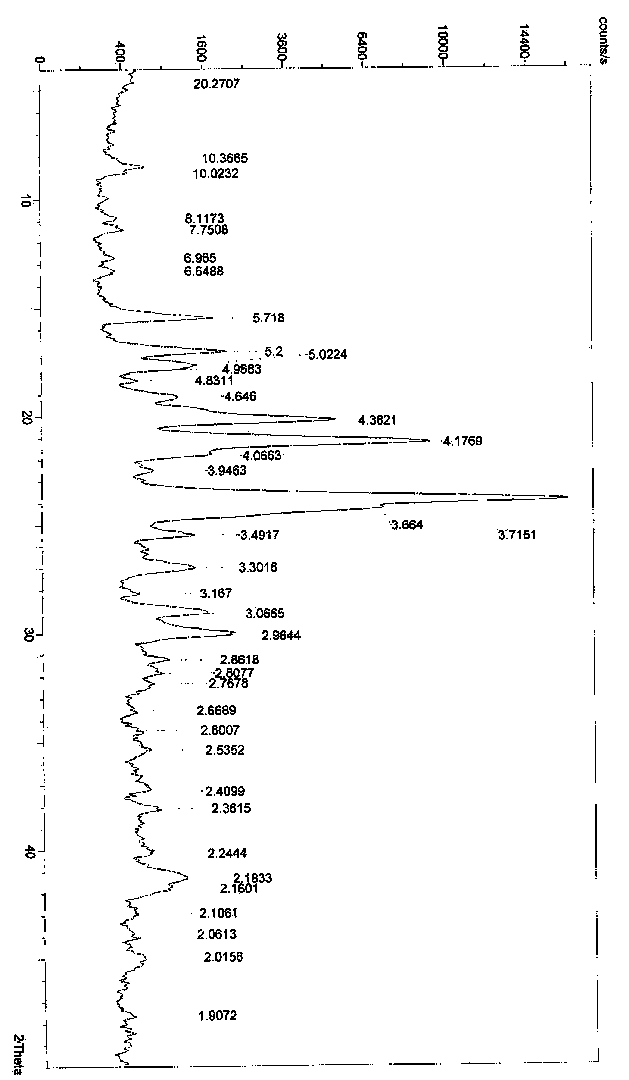
[0061] Embodiment 3: Preparation of ingavirine crystal form B
[0062] Dissolve 2 g of ingavirin solid with 28 mL of water / ethanol (1:1), stir at room temperature for 24 h, filter, and wash the filter cake twice with ethanol to obtain 1.2 g of ingavirin crystal form B. Yield: 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com