Patents
Literature
78 results about "Polymorphanisini" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
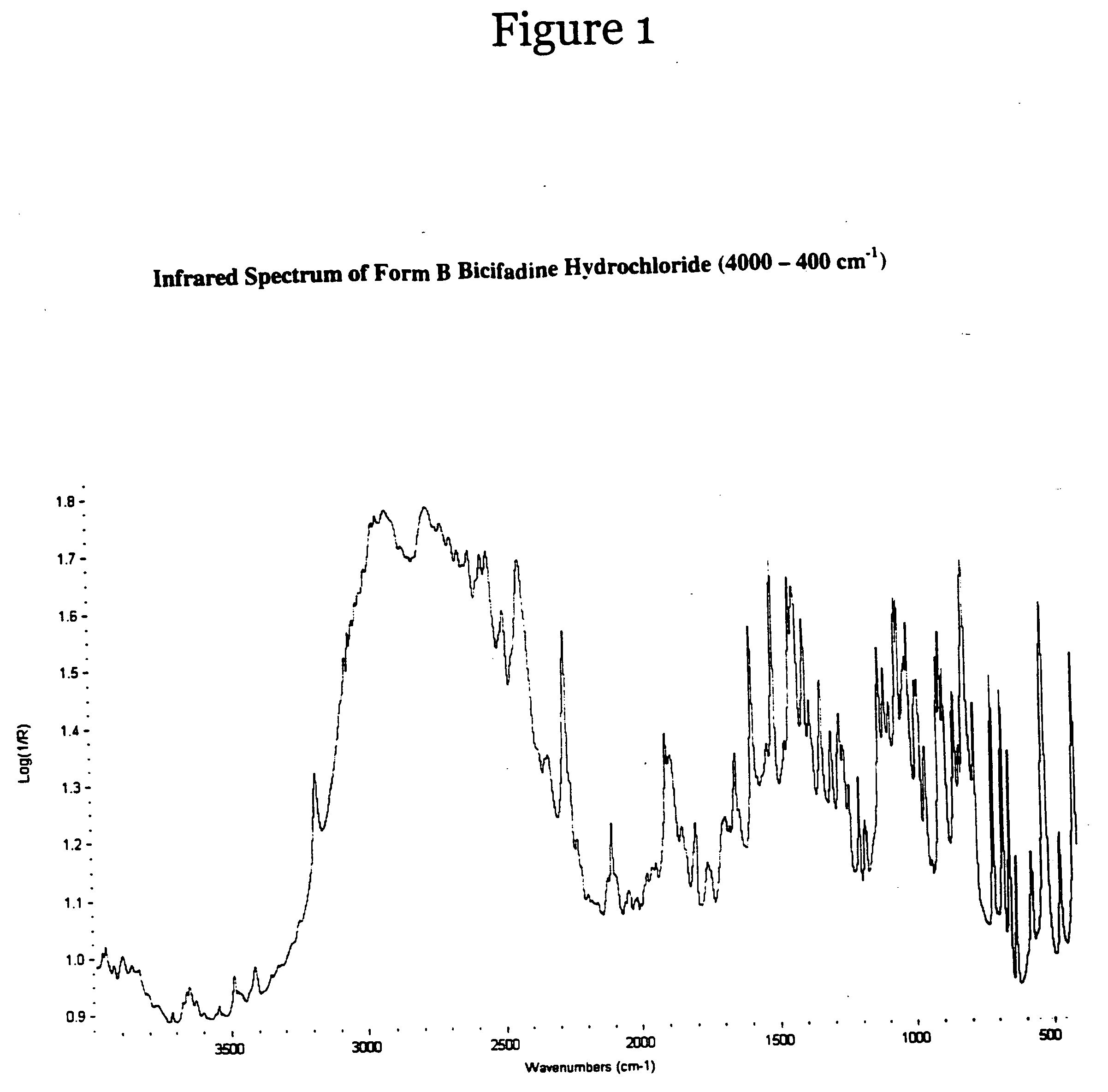
Polymorphs of bicifadine hydrochloride
A new polymorphic crystalline form of bicifadine hydrochloride, designated form B, which is more thermodynamically stable than the previously known polymorphic form of bicifadine hydrochloride, designated as form A, methods for preparing said crystalline form B and pharmaceutical compositions containing said crystalline form B.
Owner:EBI LIFE SCI INC
Use of polyols to obtain stable polymorphous forms of rifaximin
Polyols stabilize polymorphous form of rifaximin, in particular the β form. When polyols having at least two hydroxy groups are added to rifaximin powder, polymorph β is stable and remains stable in time independently from the environment humidity. In this invention a method to prepare formulations constituted by pure and stable polymorphous forms able to give a pharmaceutical product is described.
Owner:ALFASIGMA SPA
Preparation of psilocybin, different polymorphic forms, intermediates, formulations and their use
ActiveUS20190119310A1Organic active ingredientsOrganic compounds purification/separation/stabilisationMedicineBiology
This invention relates to the large-scale production of psilocybin for use in medicine. More particularly, it relates to a method of obtaining high purity crystalline psilocybin, particularly, in the form of Polymorph A. It further relates to a method for the manufacture of psilocybin and intermediates in the production thereof and formulations containing psilocybin.
Owner:COMPASS PATHFINDER LTD
Polymorph form II of tanaproget
Tanaproget polymorph Form II, processes for preparing tanaproget polymorph Form II, pharmaceutical compositions including tanaproget polymorph Form II, micronized tanaproget polymorph Form II, and processes for converting Form II to tanaproget Form I are provided. Also provided are methods of contraception, hormone replacement therapy, stimulation of food intake and treating or preventing uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, or carcinomas and adenocarcinomas comprising administering polymorph Form II to a mammalian subject.
Owner:WYETH LLC
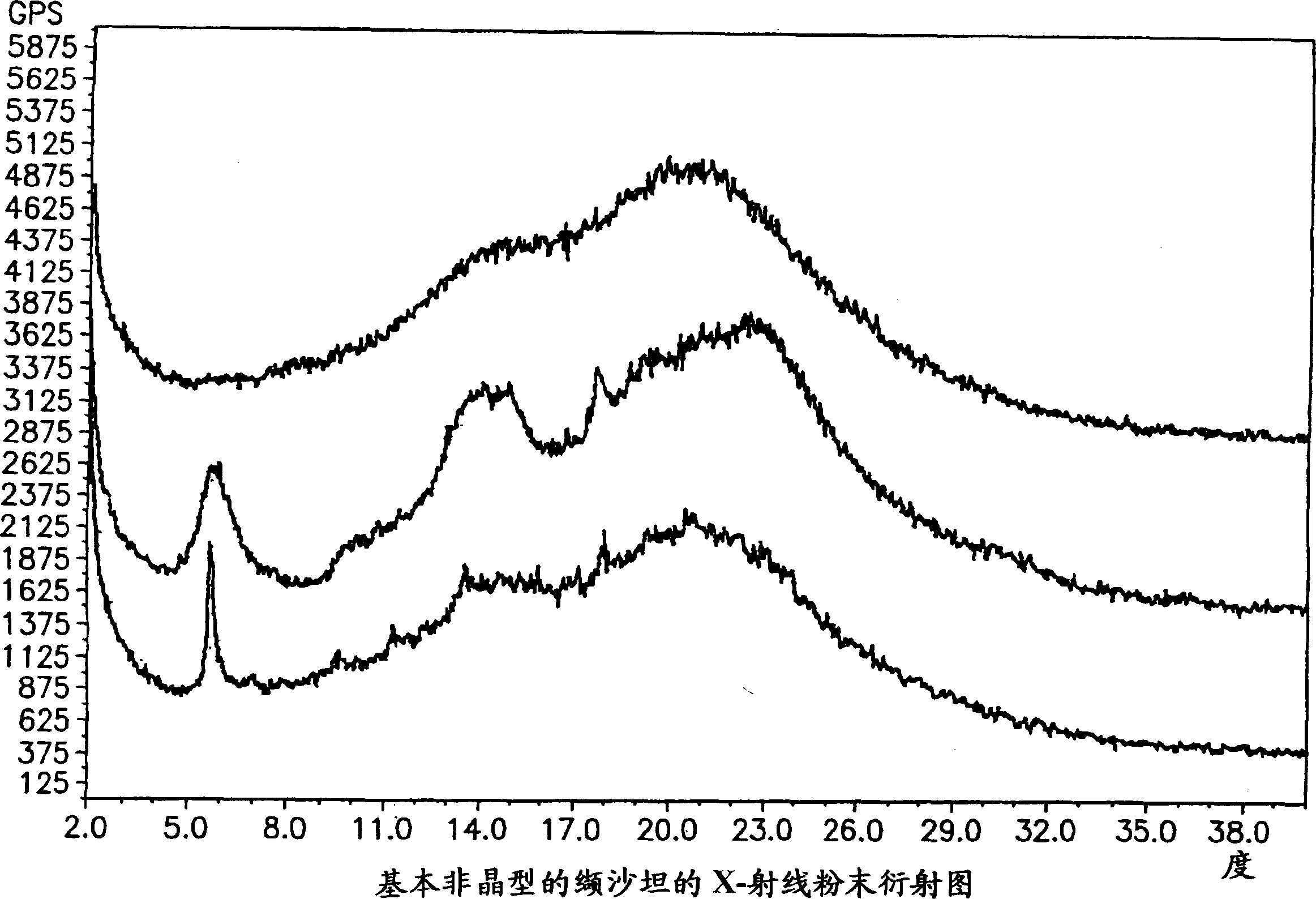
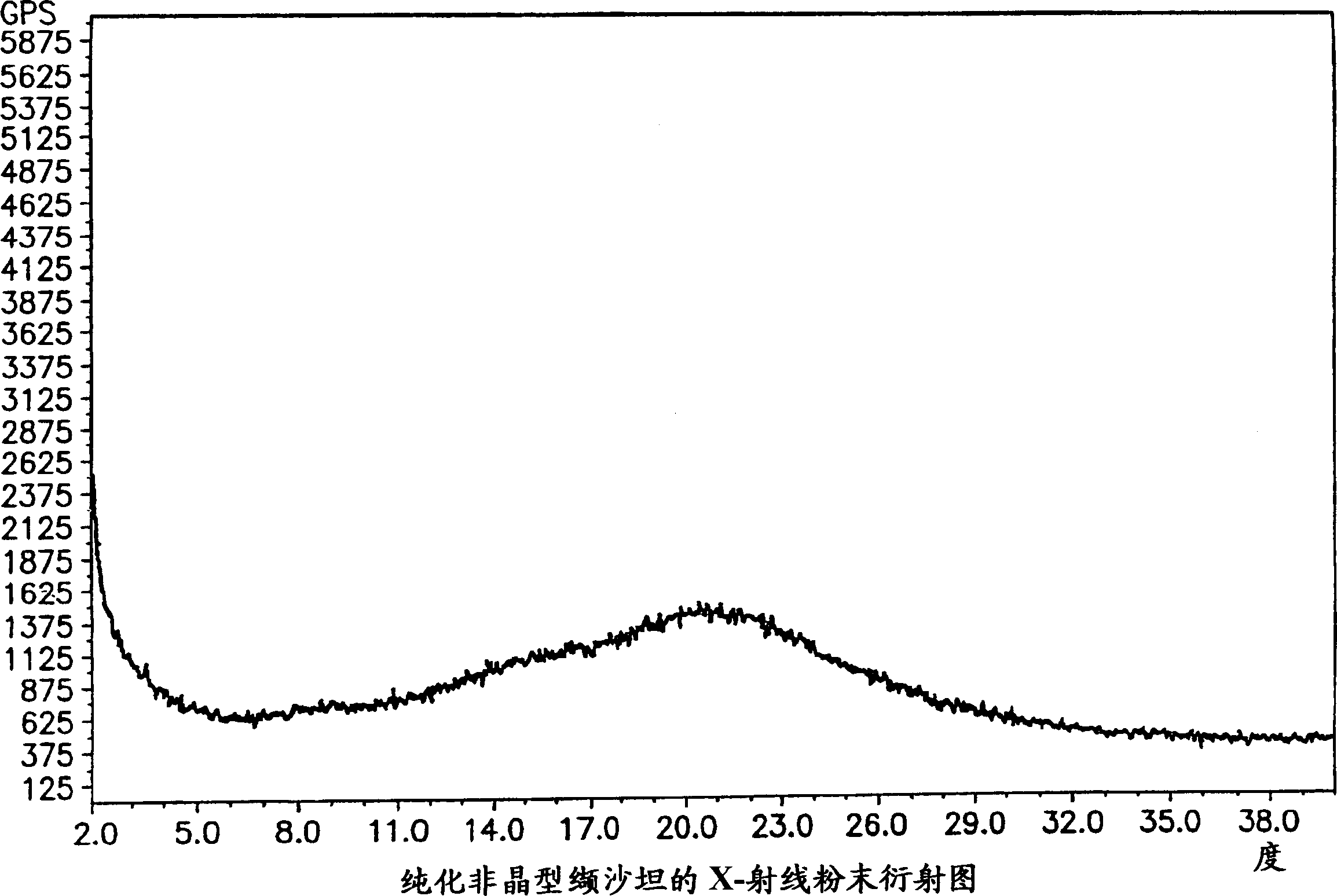
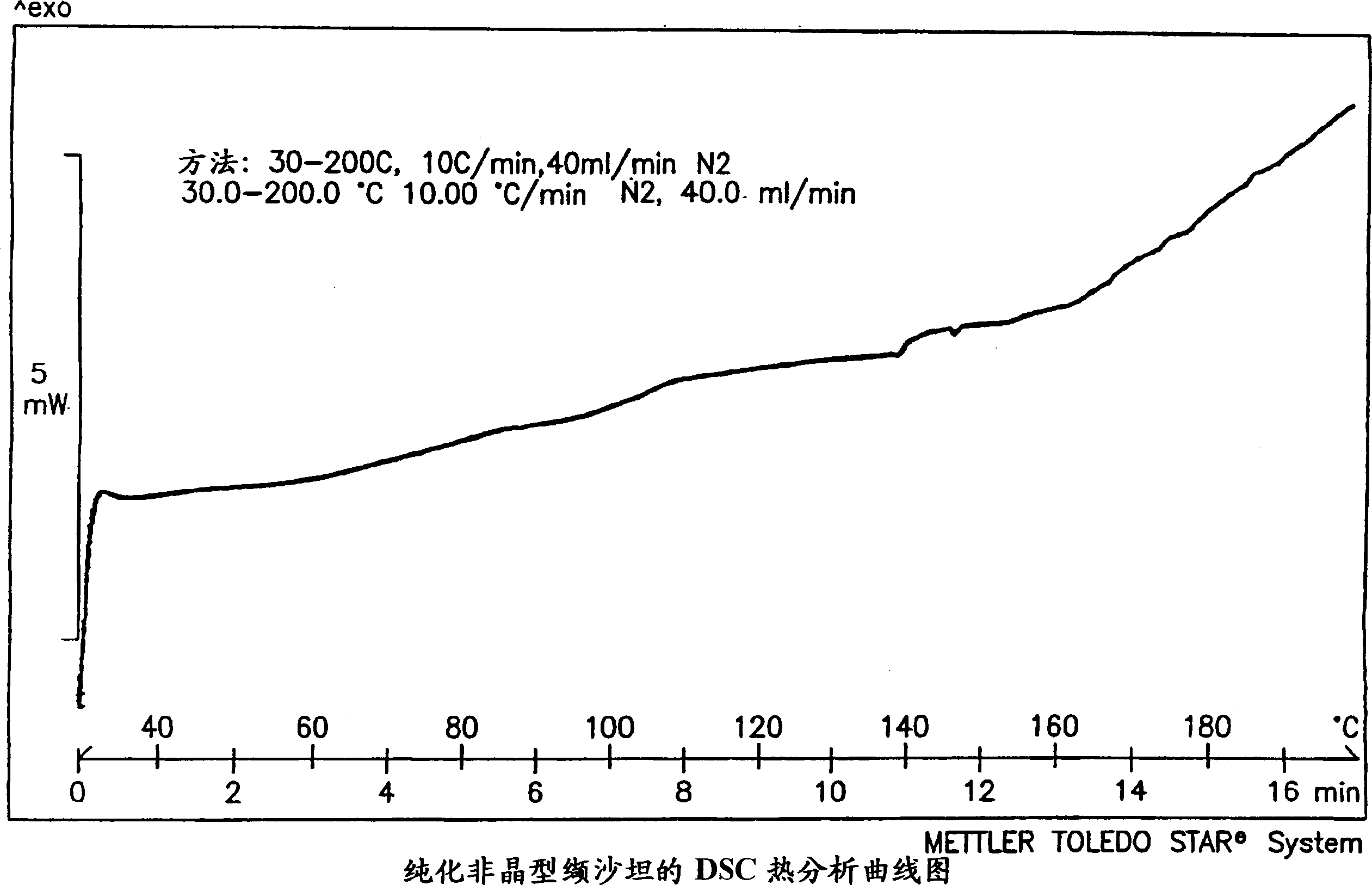
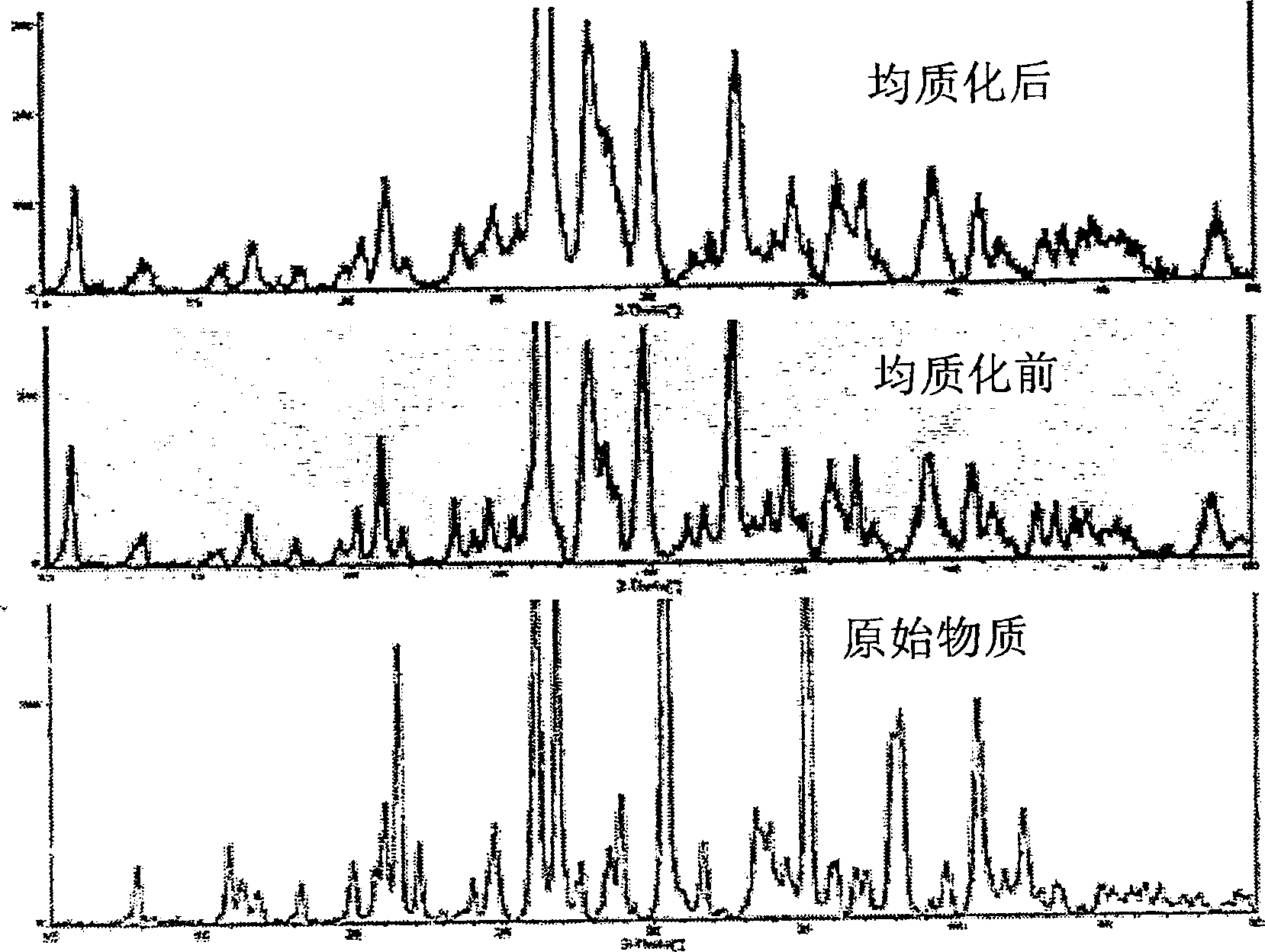
Polymorphis of valsartan
Owner:TEVA PHARMA IND LTD
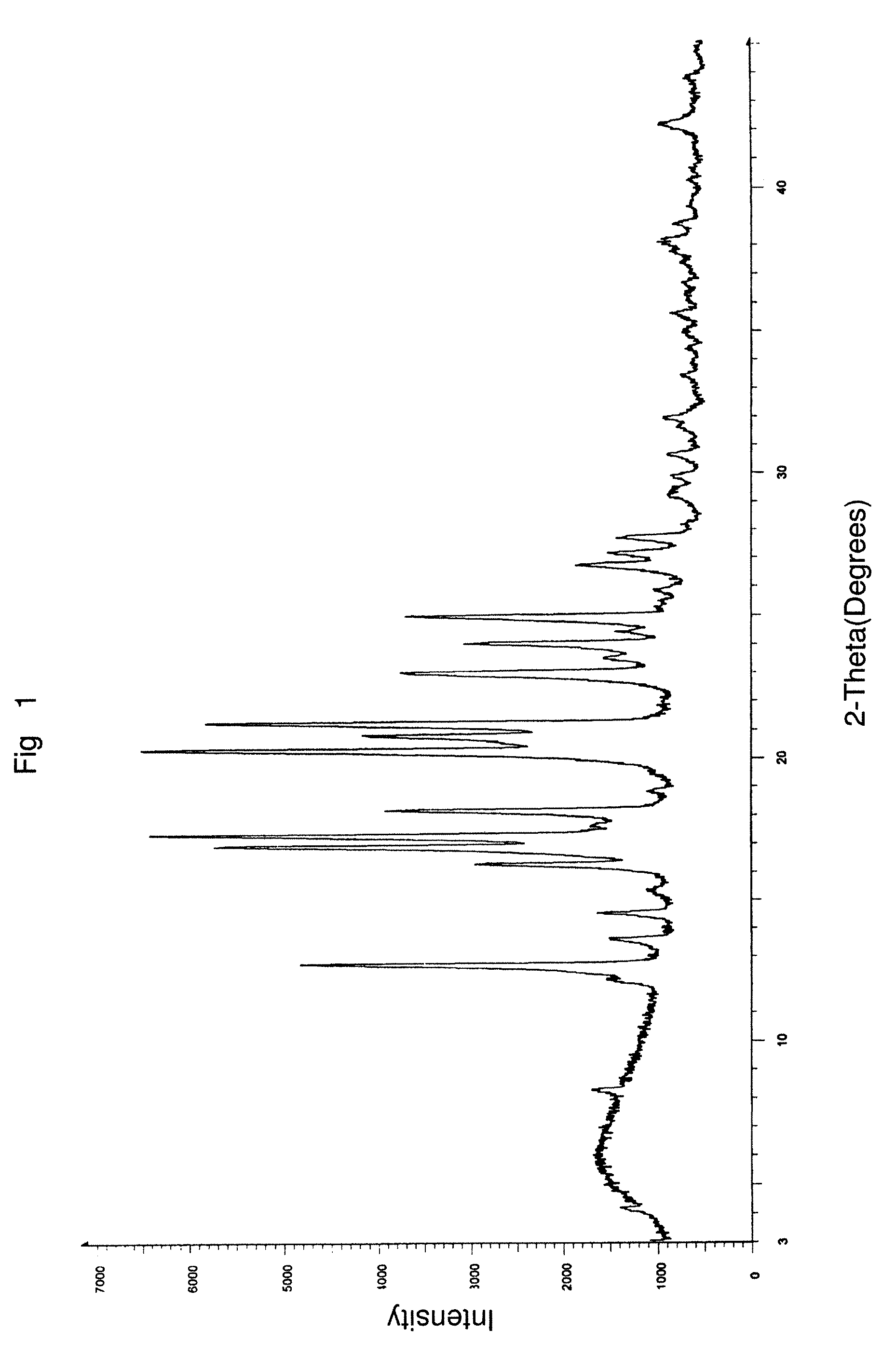
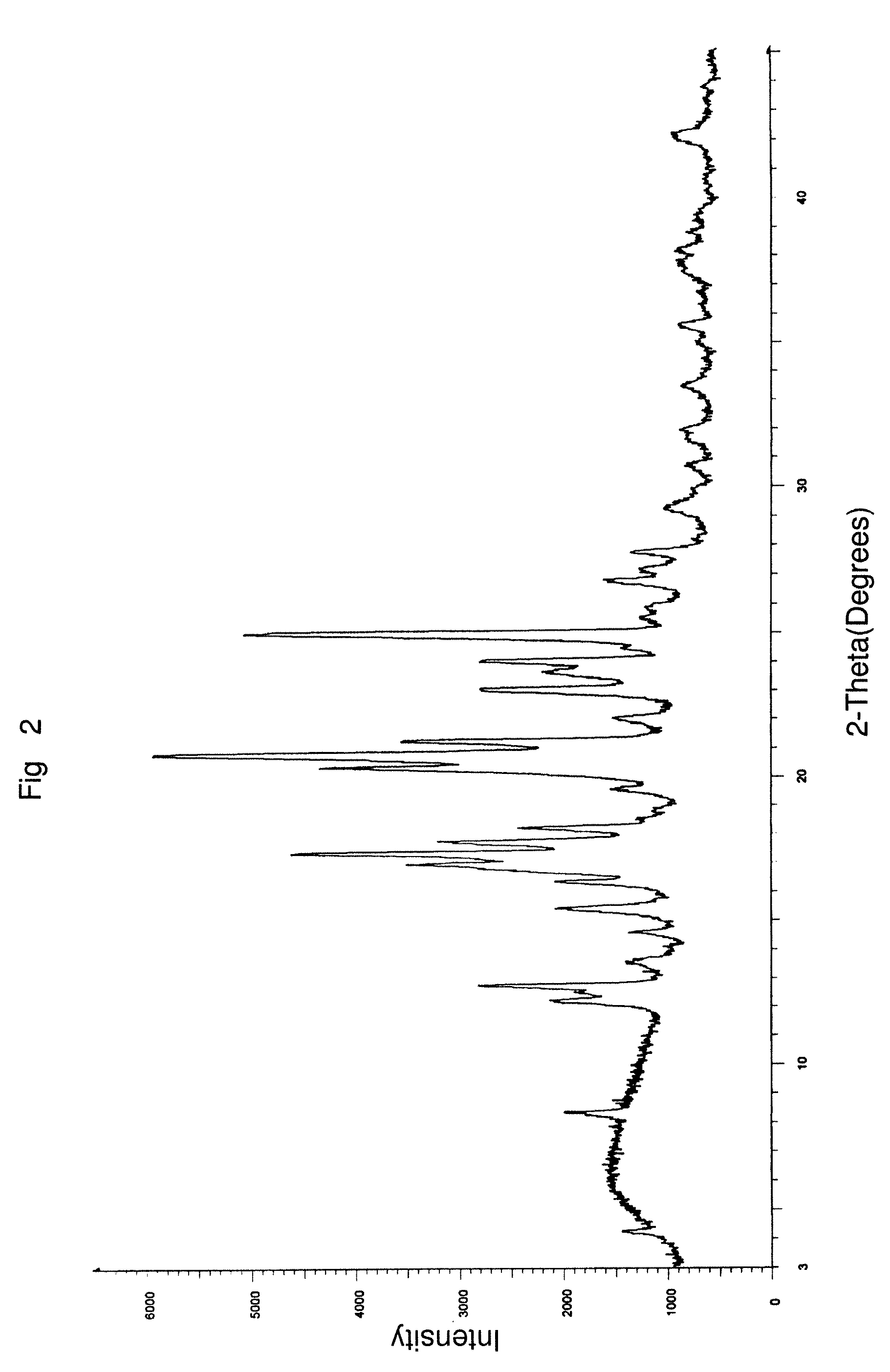
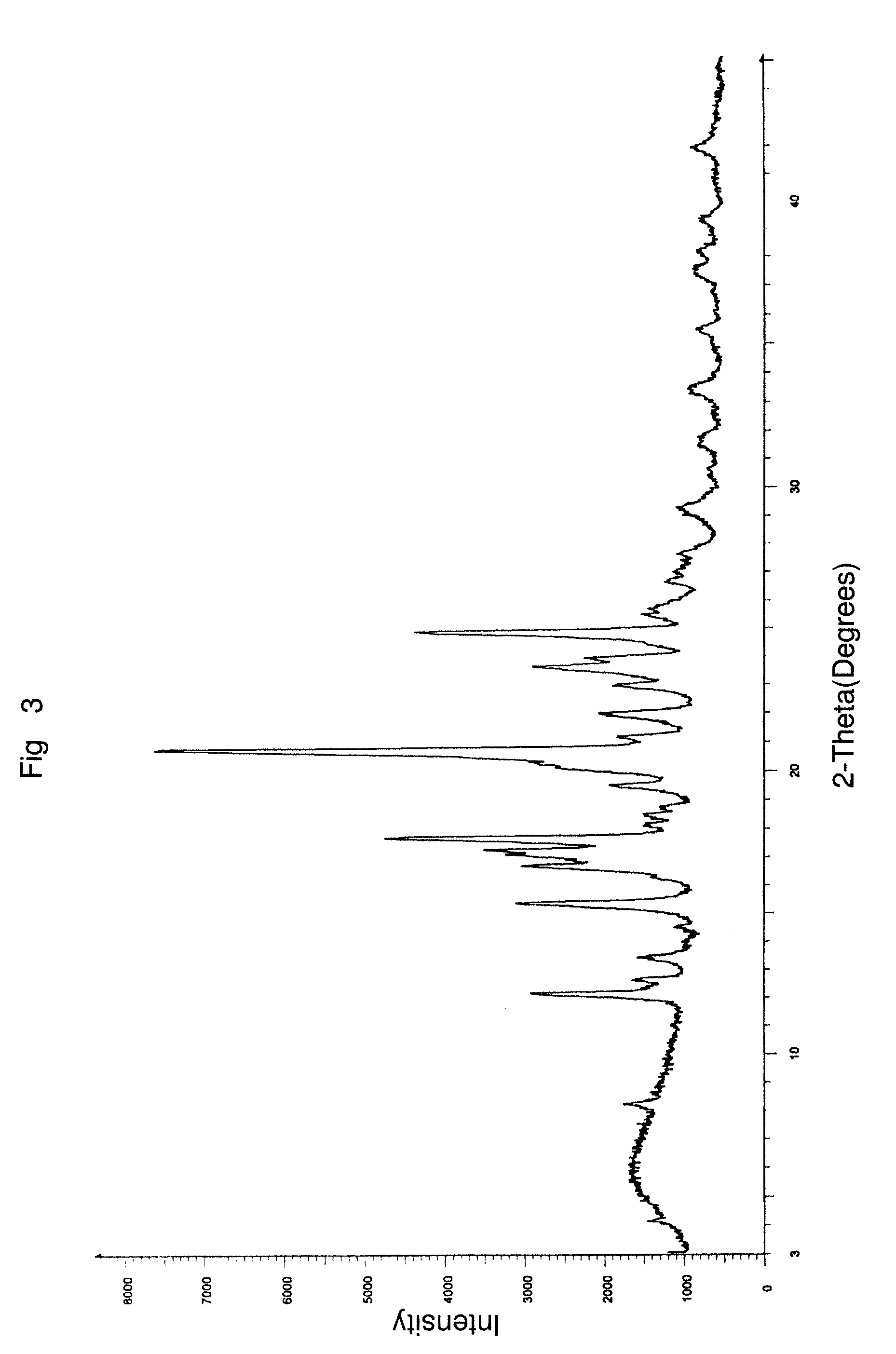
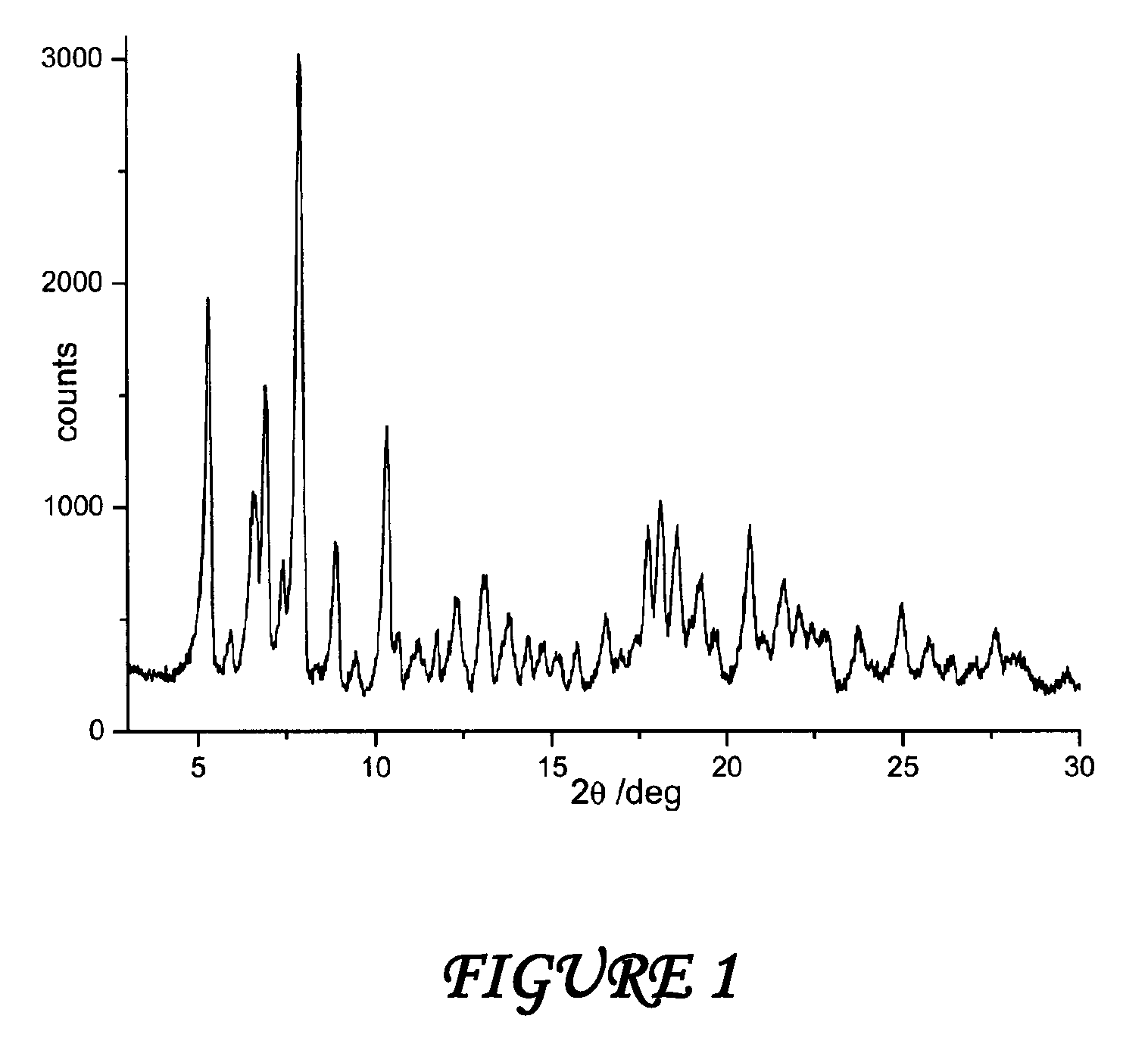
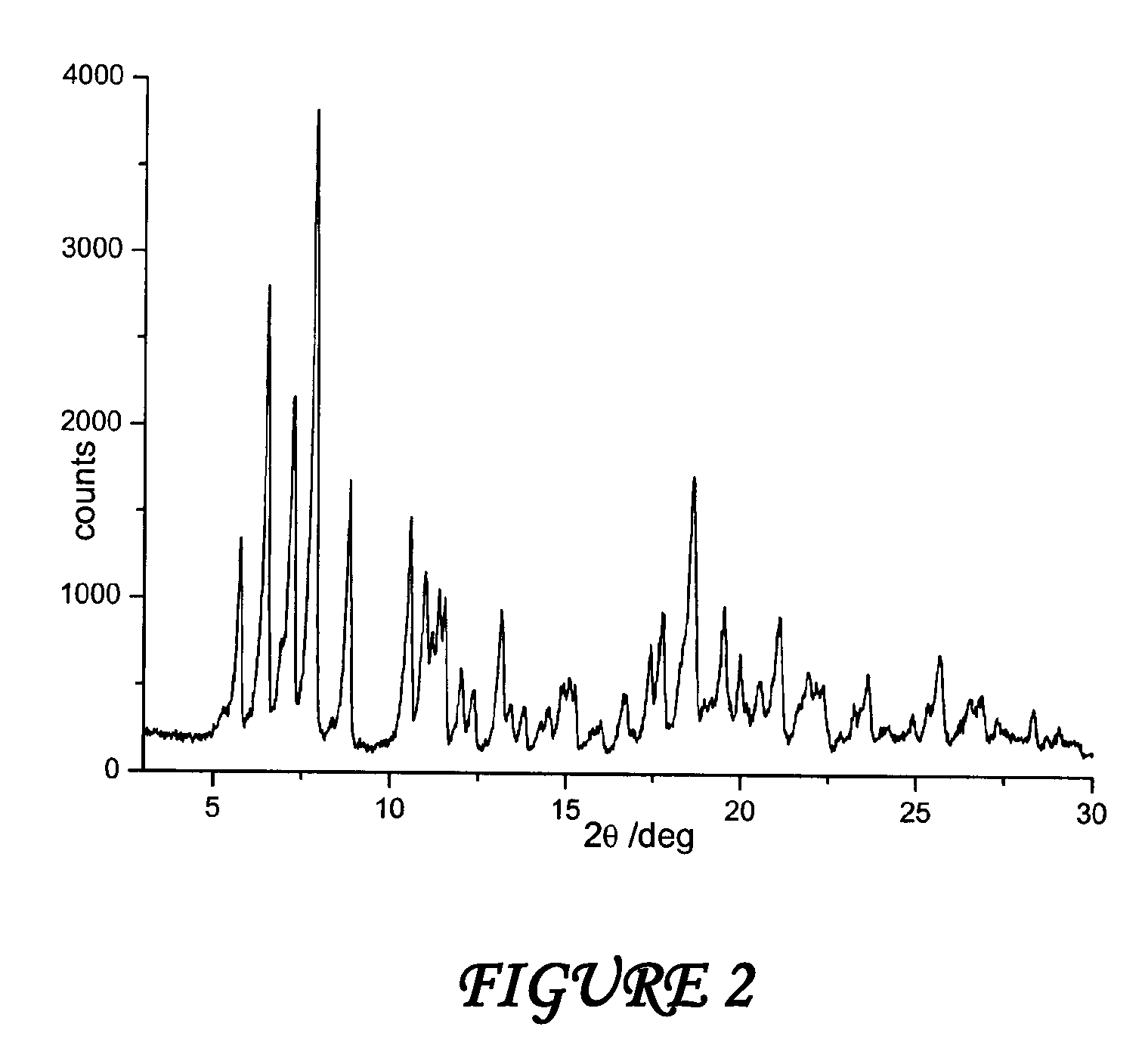
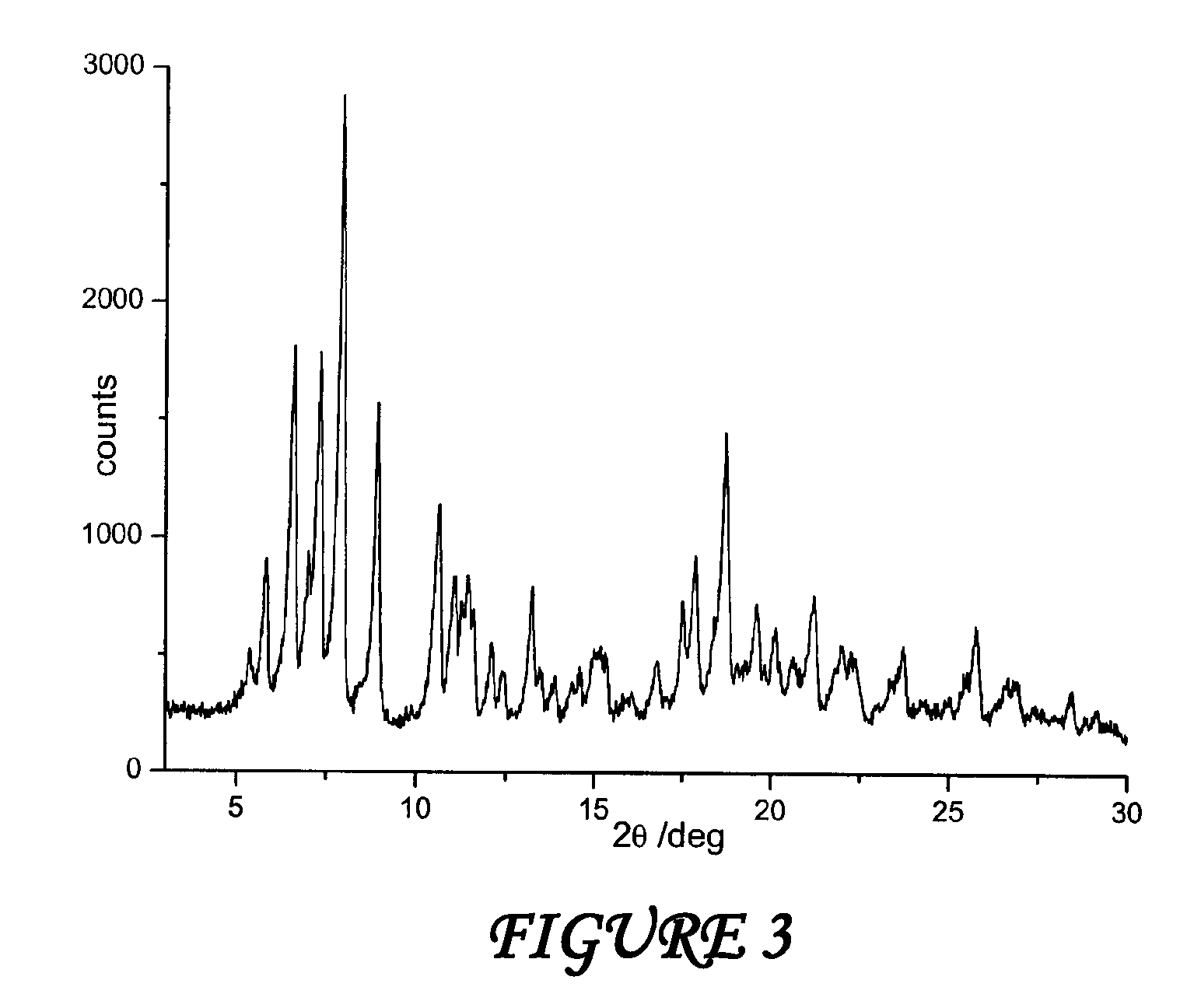
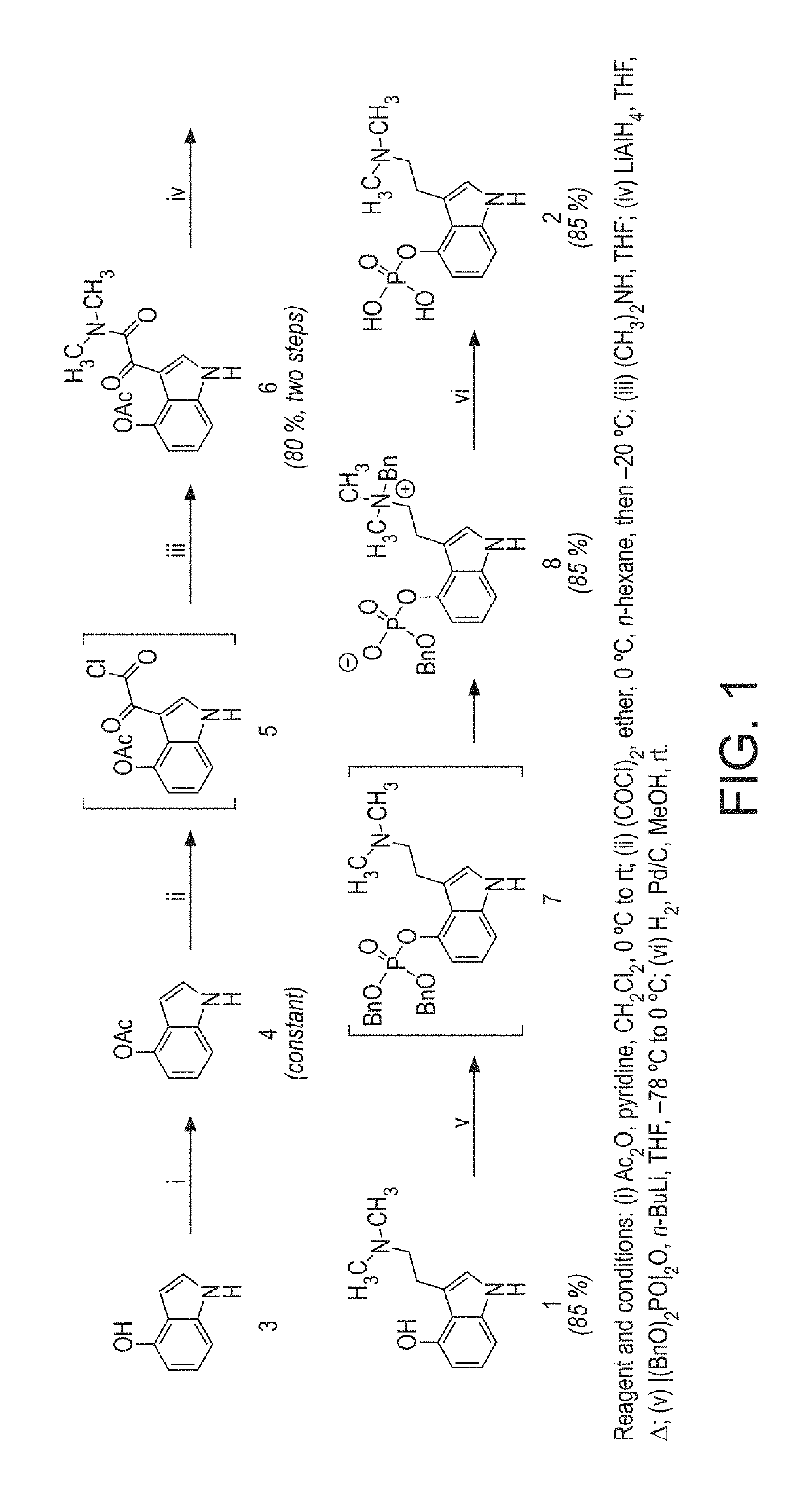
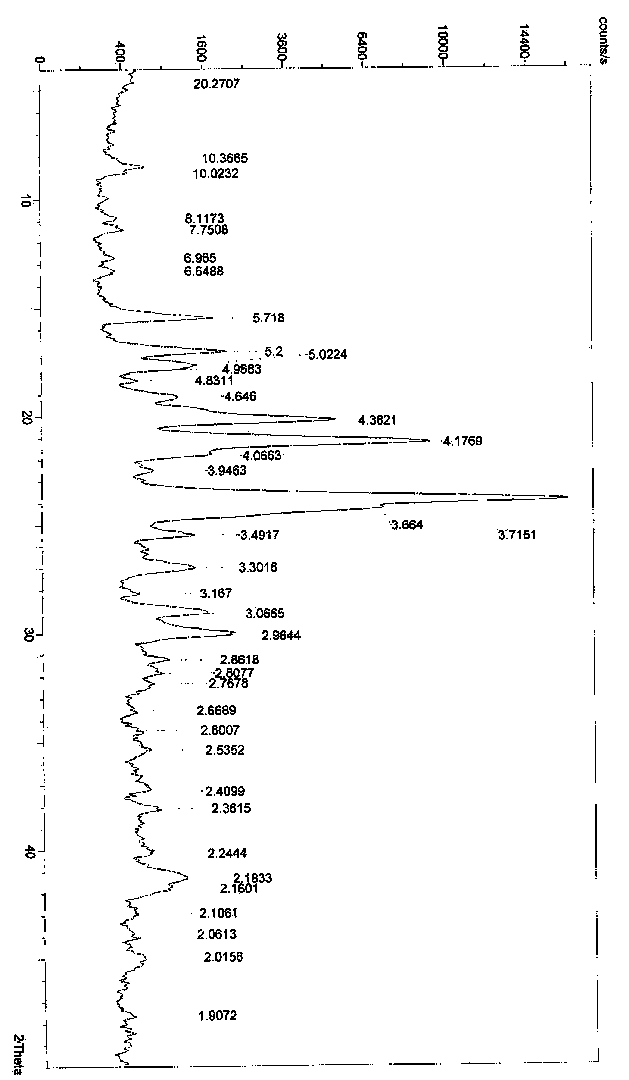
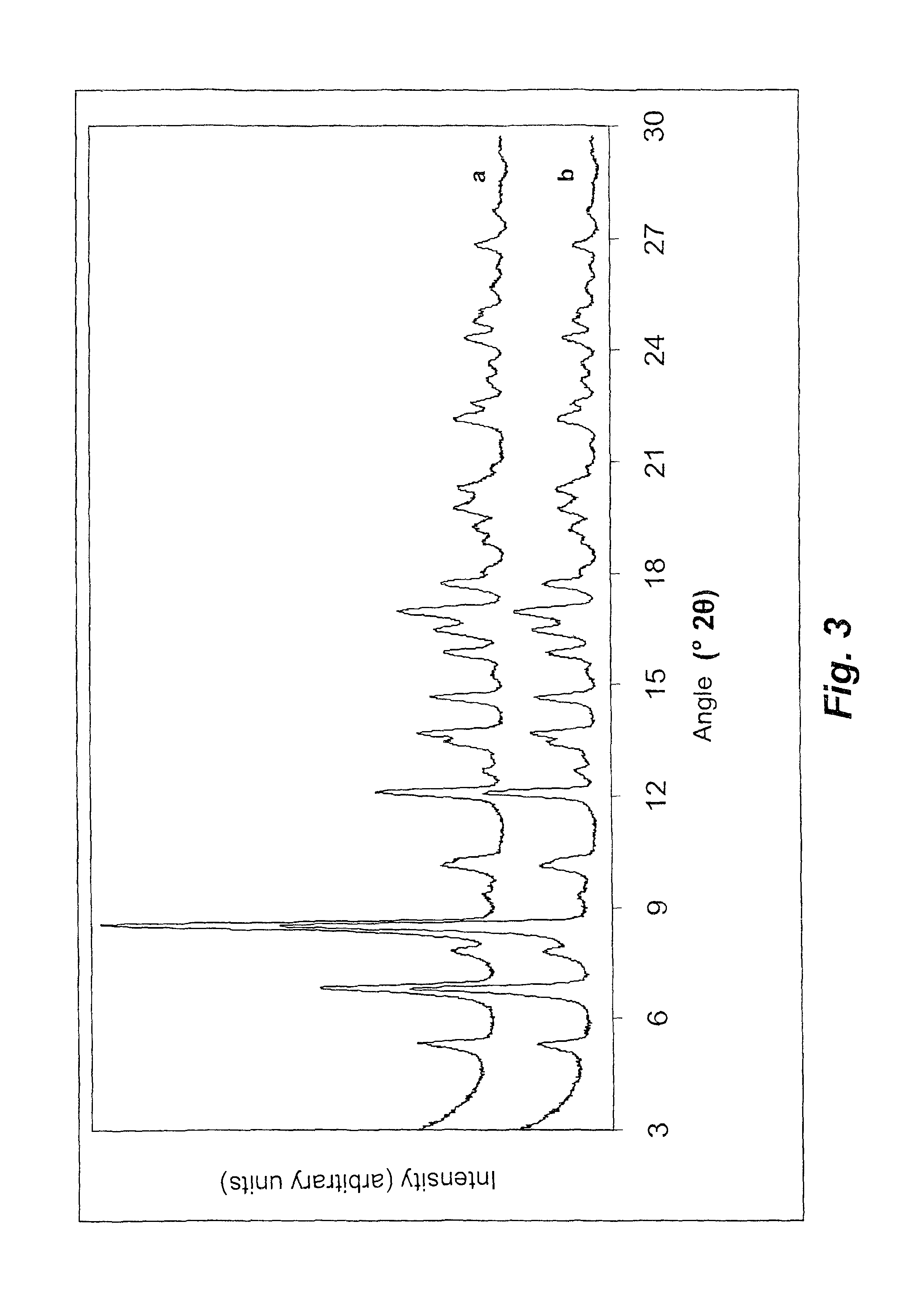
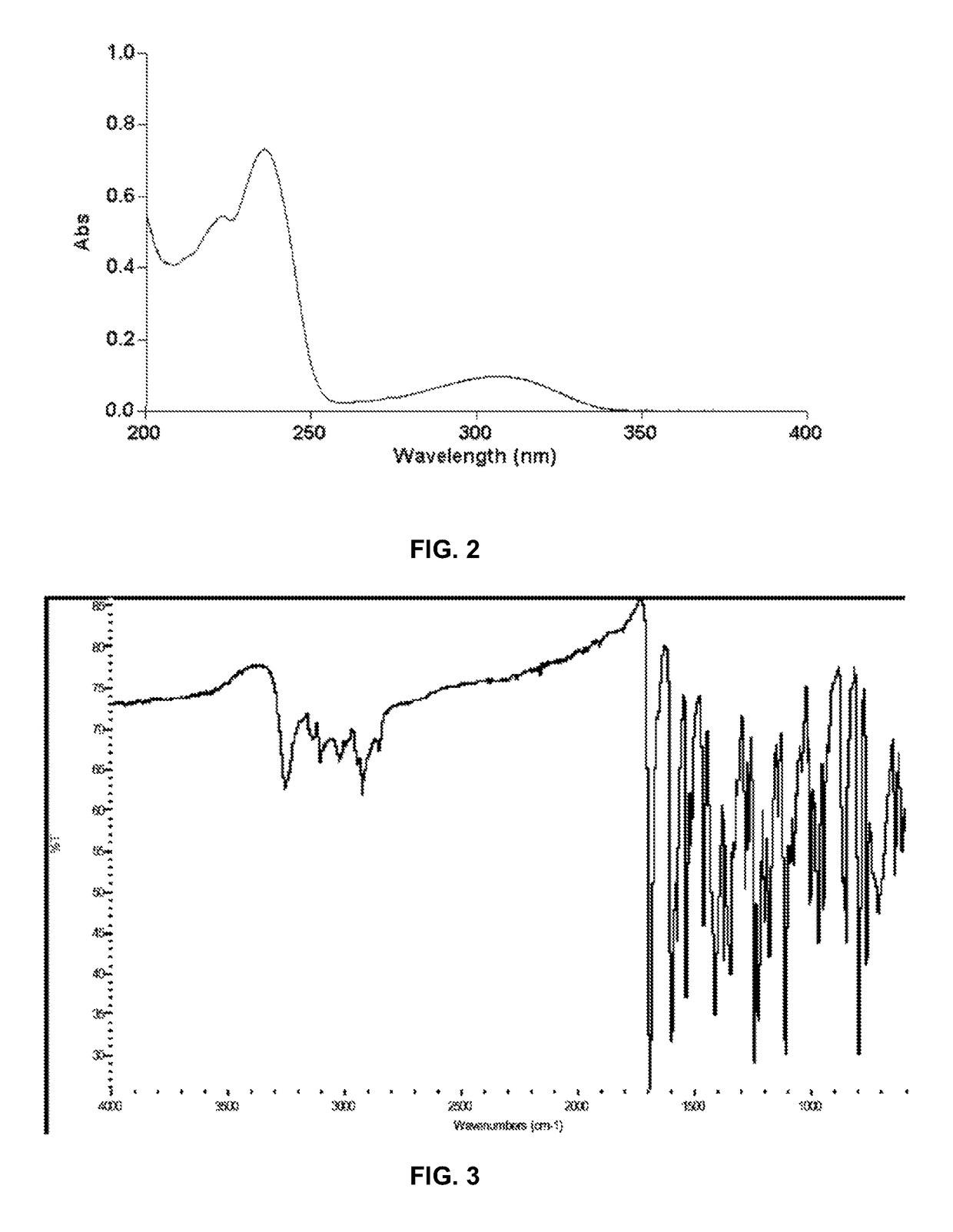
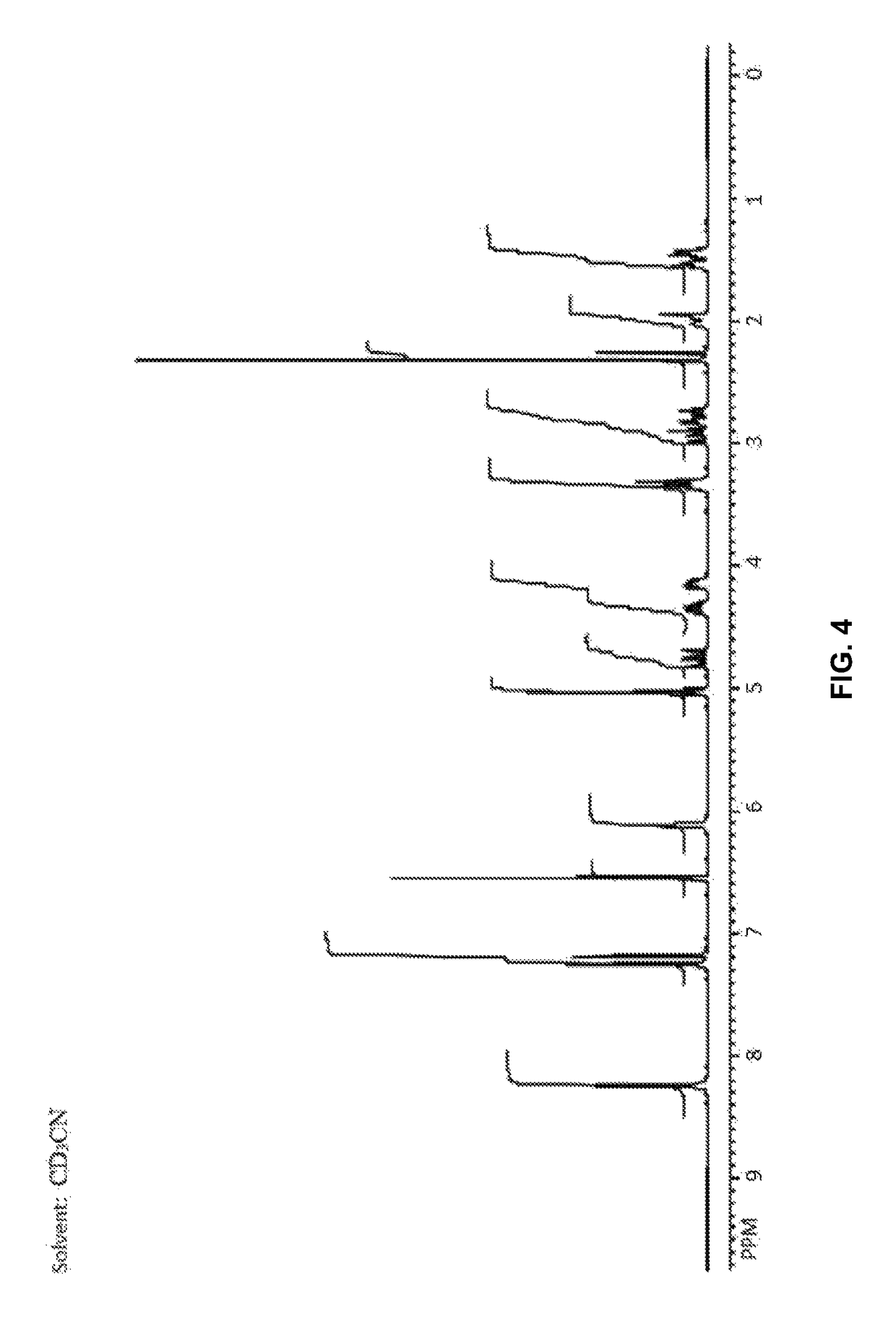
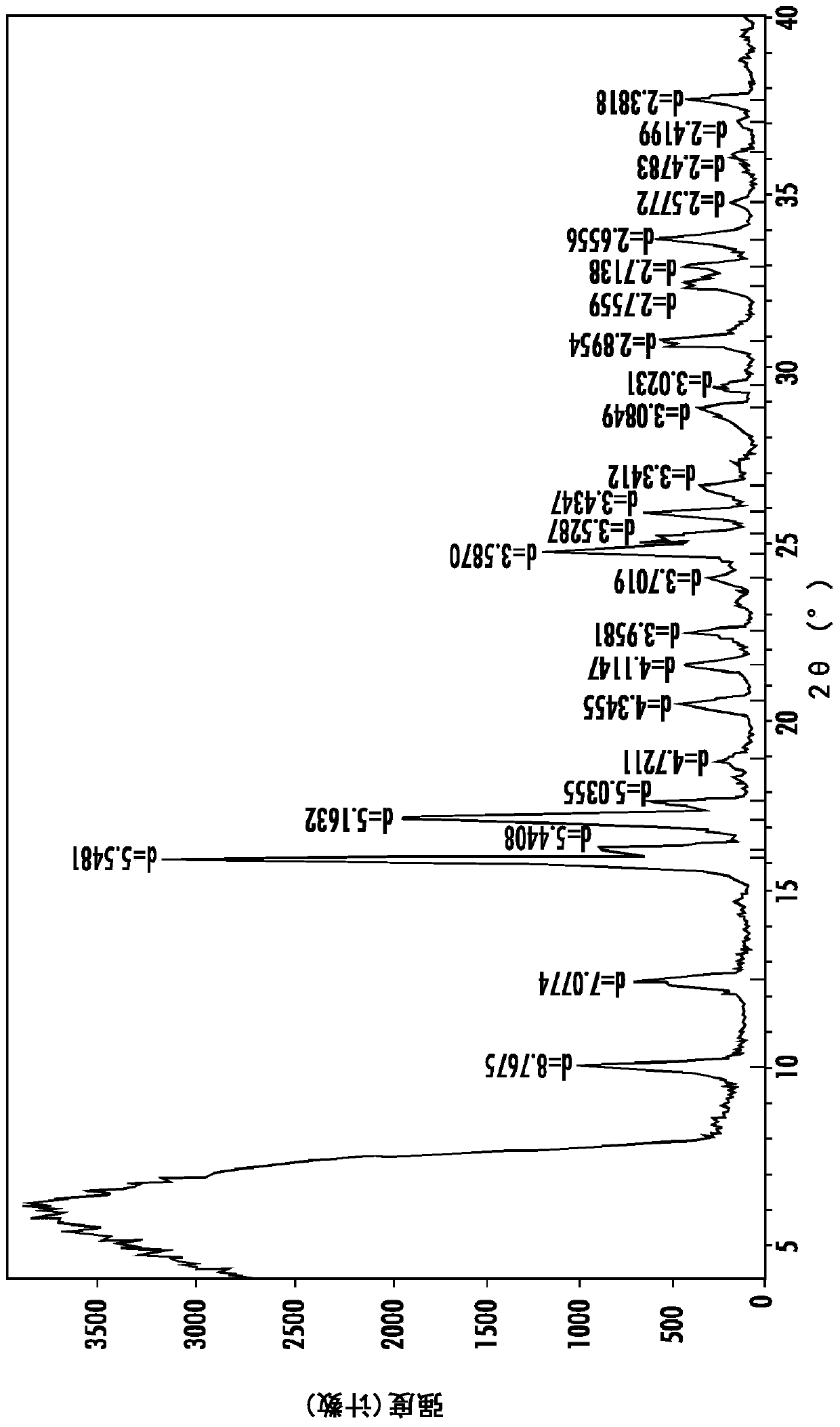
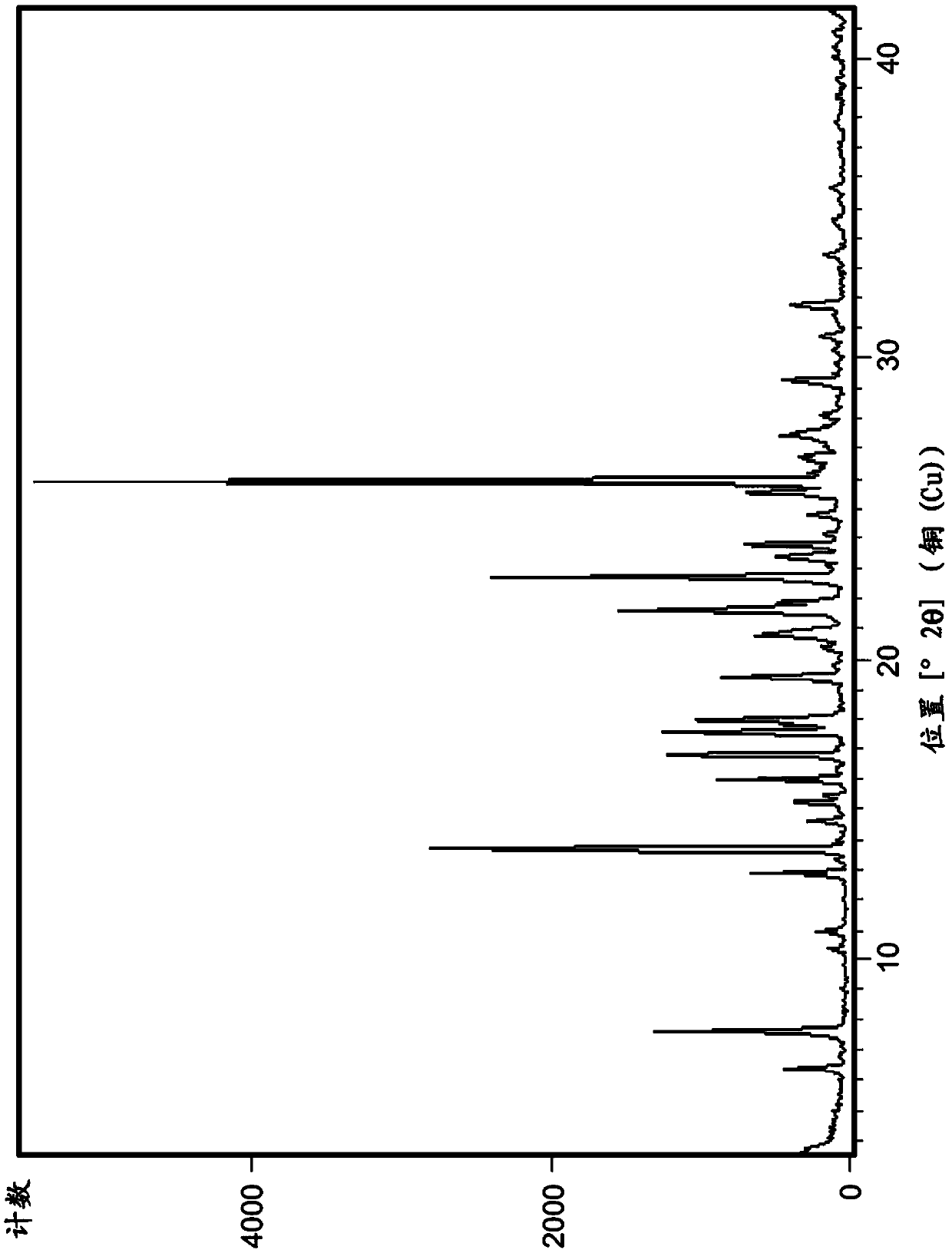
Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto
Polymorph Form III of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-α]-pyrimidin-7-yl}phenyl)acetamide, and use thereof as a sedative-hypnotic, anxiolytic, anticonvulsant, and / or skeletal muscle relaxant agent. Related compositions and methods are also disclosed, particularly with regard to treatment of insomnia.
Owner:NEUROCRINE BIOSCI INC
Preparation of submicron sized particles with polymorph control and new polymorph of itraconazole
The present invention provides a method of preparing particles with polymorph and size control of a pharmaceutical compound, the method including the steps of: (1) providing pharmaceutical compound in a first phase; (2) seeding the compound; (3) causing a phase change in the pharmaceutical compound to a second phase of a desired polymorphic form; and (4) wherein the mean particle size of the particles is less than 7 mum. The present invention further provides a polymorphic form of itraconazole.
Owner:BAXTER INT INC
Histone deacetylase inhibitors
InactiveCN101896457AOrganic chemistryOrganic compound preparationMetabolitePharmaceutical Substances
Novel compounds of the general formula (I), having histone deacetylase (HDAC) inhibiting enzymatic activity, their derivatives, analogs, tautomeric forms, stereoisomers, polymorphs, hydrates, solvates, intermediates, pharmaceutically acceptable salts, pharmaceutical compositions, metabolites and prodrugs thereof. The present invention more particularly provides novel compounds of the general formula (I). Also included is a method for treatment of cancer, psoriasis, proliferative conditions and conditions mediated by HDAC, in a mammal comprising administering an effective amount of a novel compound of formula (I).
Owner:ORCHID RES LAB
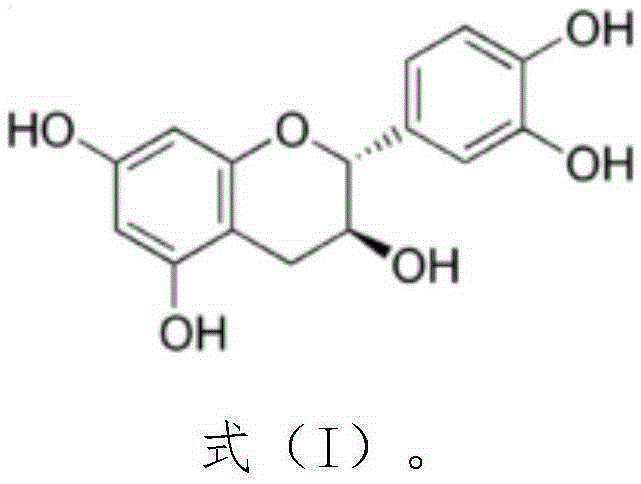
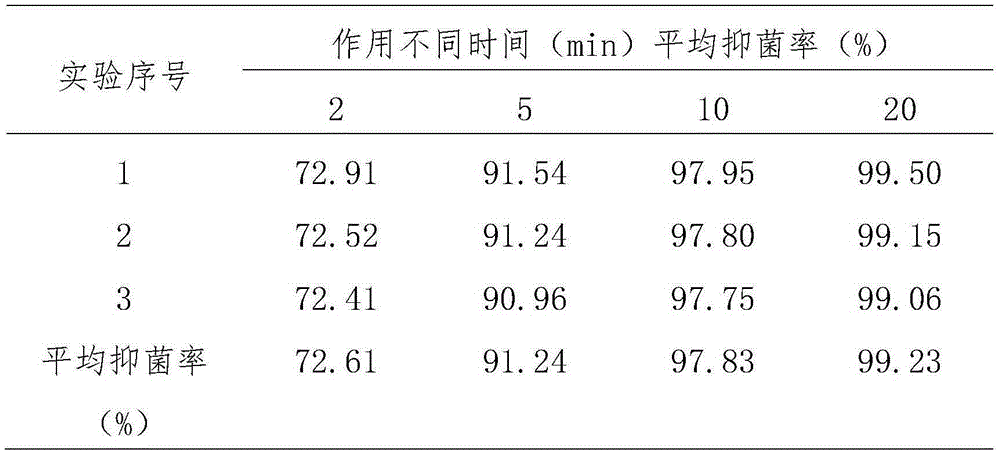
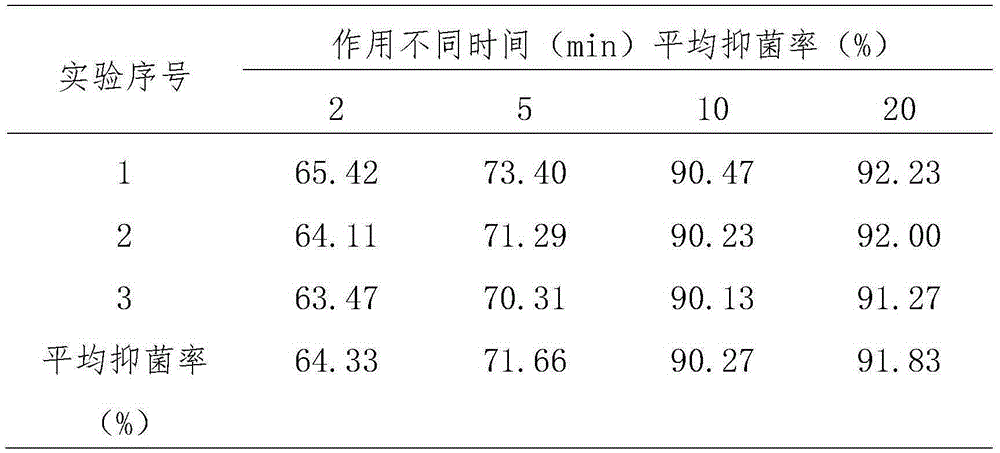
Application of medicine containing catechin to preparation of antibacterial medicines
ActiveCN105232526ABroad spectrum antibacterialNon-irritatingAntibacterial agentsOrganic active ingredientsEscherichia coliIrritation
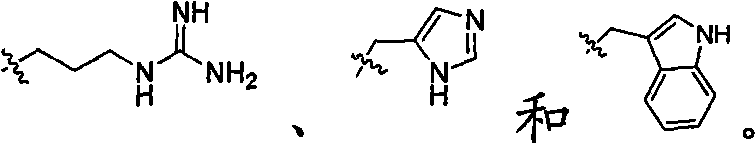
The invention provides application of a medicine containing catechin shown in formula (I) or catechin polymorphs to preparation of antibacterial medicines. Through experiment research, the catechin is wide in antibacterial spectrum and has exact inhibiting effect on various pathogenic microorganisms such as staphylococcus aureus, escherichia coli, candida albicans, pseudomonas aeruginosa and hemolytic streptococcus; meanwhile, the catechin has good selectivity and has no irritation to normal flora and vagina mucosa, thereby being promising in application prospect on preparation of the antibacterial medicines. Formula (I).
Owner:王孝仓
Ingavirin polymorph and its preparation method
InactiveCN103382179AGood physical and chemical propertiesImprove stabilityOrganic chemistryEngineeringIn vivo
The invention belongs to the field of chemical pharmaceutical technology and relates to an ingavirin polymorph and its preparation method. The invention aims to provide the ingavirin polymorph in allusion to insufficiencies existing in the prior art. The invention provides ingavirin polymorphs A and B which have excellent physicochemical properties and good stability and are used in the research on in vivo study of ingavirin. Meanwhile, the invention provides a preparation method of the polymorphs A and B.
Owner:SICHUAN BAILI PHARM CO LTD
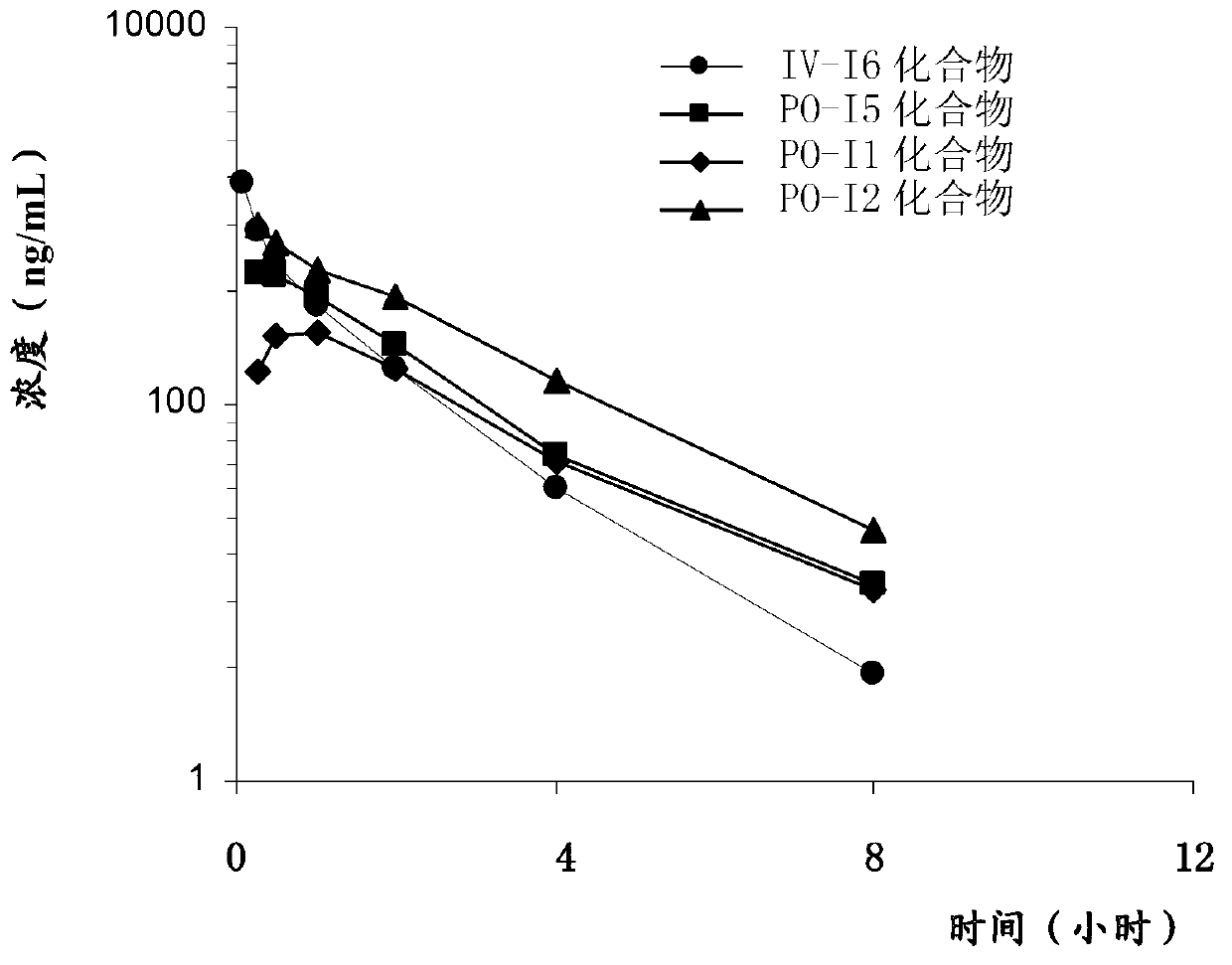
Dabigatran etexilate derivative and preparation method and application thereof
ActiveCN102766134AImprove oral bioavailabilityStrong anticoagulant inhibitory effectOrganic active ingredientsOrganic chemistryRacemic mixtureDabigatran
The invention provides a dabigatran etexilate derivative shown as a formula I, or pharmaceutically acceptable salt, a solvate, a polymorph, an antipode or a racemic mixture thereof. In the formula I, R1 is hydrogen or C1 to C5 alkyl, R2 is shown in the specifications, R3 and R4 are independently hydrogen or C1 to C5 alkyl, n is 0 or 1, and R5 is C1 to C8 alkyl or optionally substituted C1 to C8 alkyl. The compound has the activity of a thrombin inhibitor. The invention also provides a preparation method for the compound, a compound-containing medical composition, and application of the compound and the medicinal composition to preparation of thrombin inhibitor medicines and treatment of related diseases.
Owner:BEIJING PRELUDE PHARM SCI & TECH
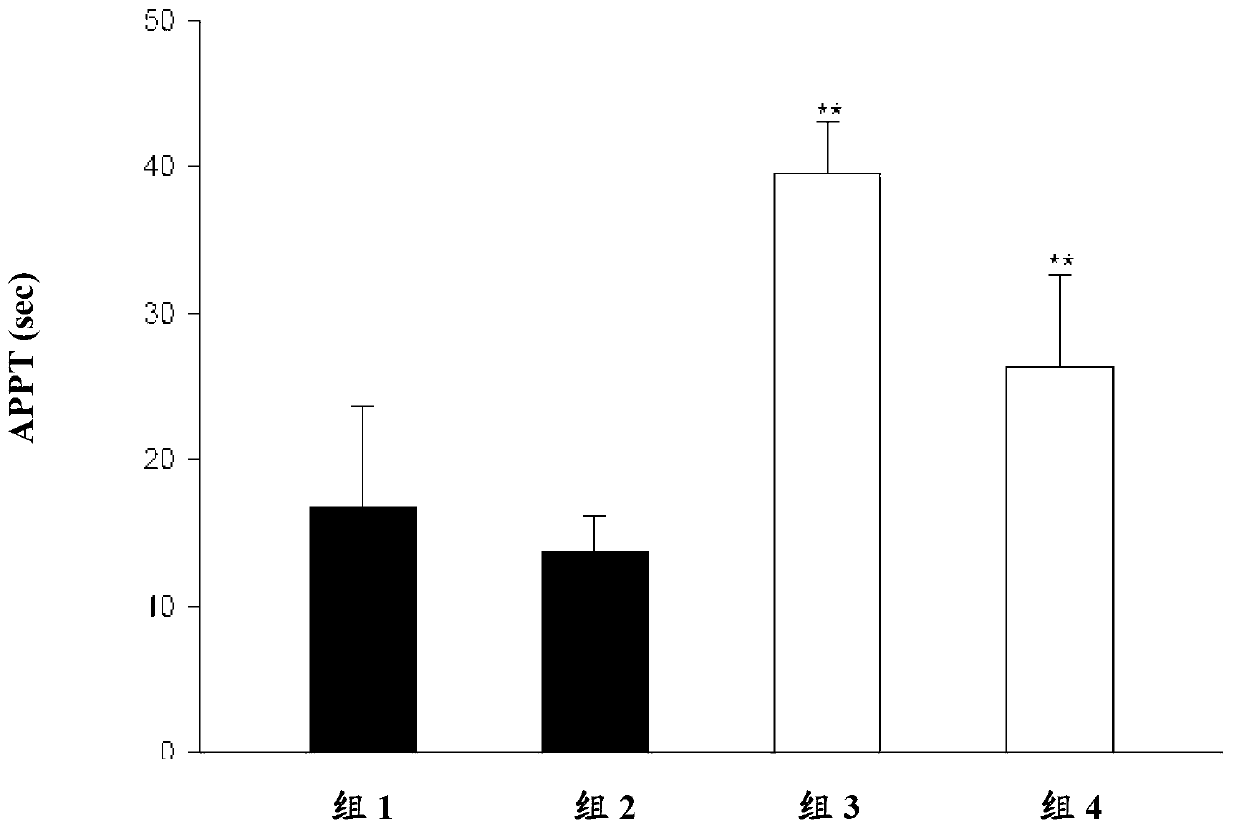
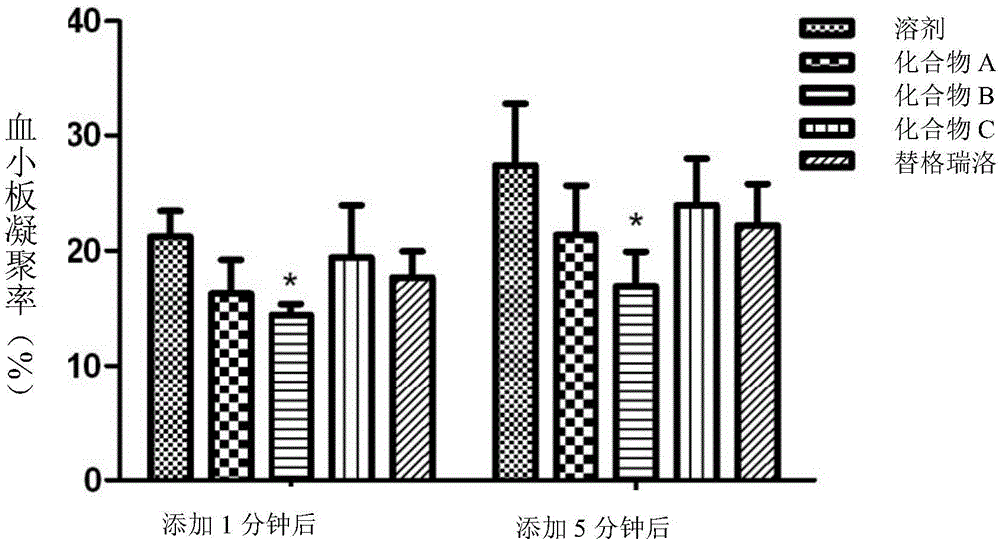
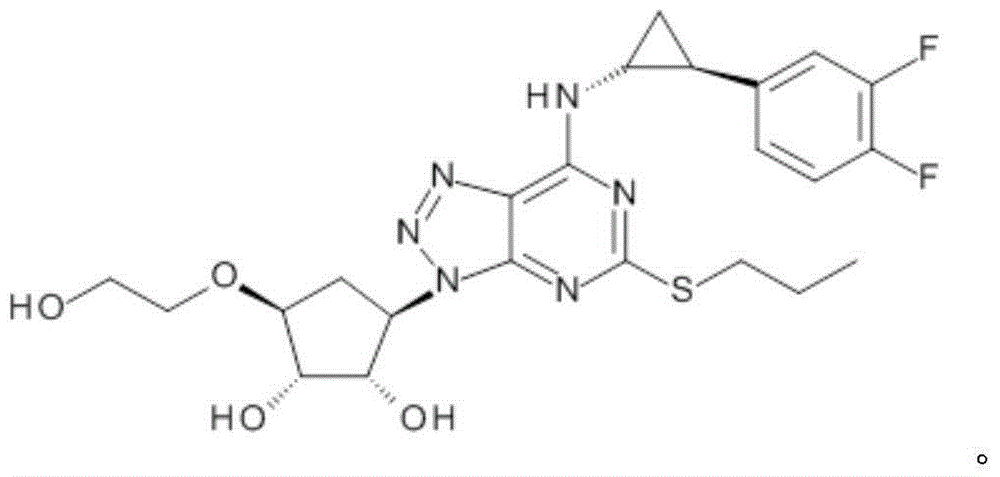
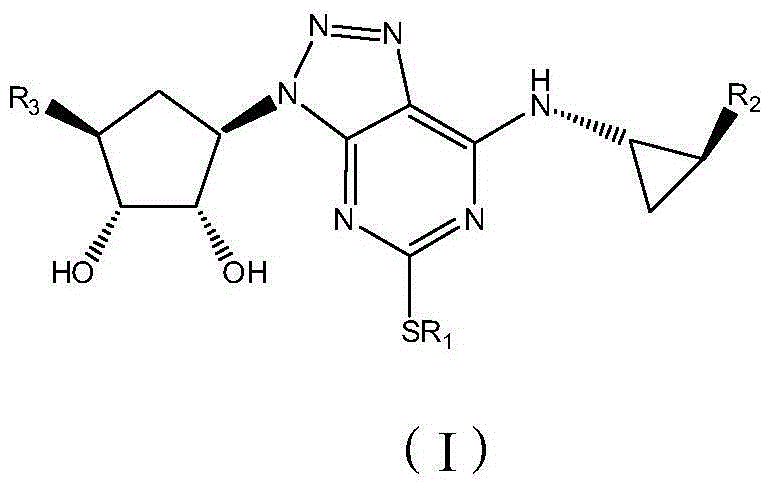
Platelet aggregation inhibitor, and preparation method and application thereof
ActiveCN105481861AGood anti-platelet aggregation effectExcellent anti-platelet aggregation effectOrganic active ingredientsOrganic chemistryHalogenPlatelet aggregation inhibitor
The invention provides a compound represented by a formula (I), or pharmaceutically acceptable salts, solvates, polymorphs, enantiomers or racemic mixtures thereof. In the formula (I), R1 is substituted or unsubstituted lower alkyl; R2 is phenyl or phenyl substituted by one or more halogen atoms; R3 is O(CH2)nR4, wherein n is an integer selected from 1-6, and R4 is OC(O)R5 or OP(O)(OH)2, wherein R5 is substituted or unsubstituted lower alkyl or R6NH2, wherein R6 is substituted or unsubstituted lower alkyl.
Owner:BEIJING PRELUDE PHARM SCI & TECH
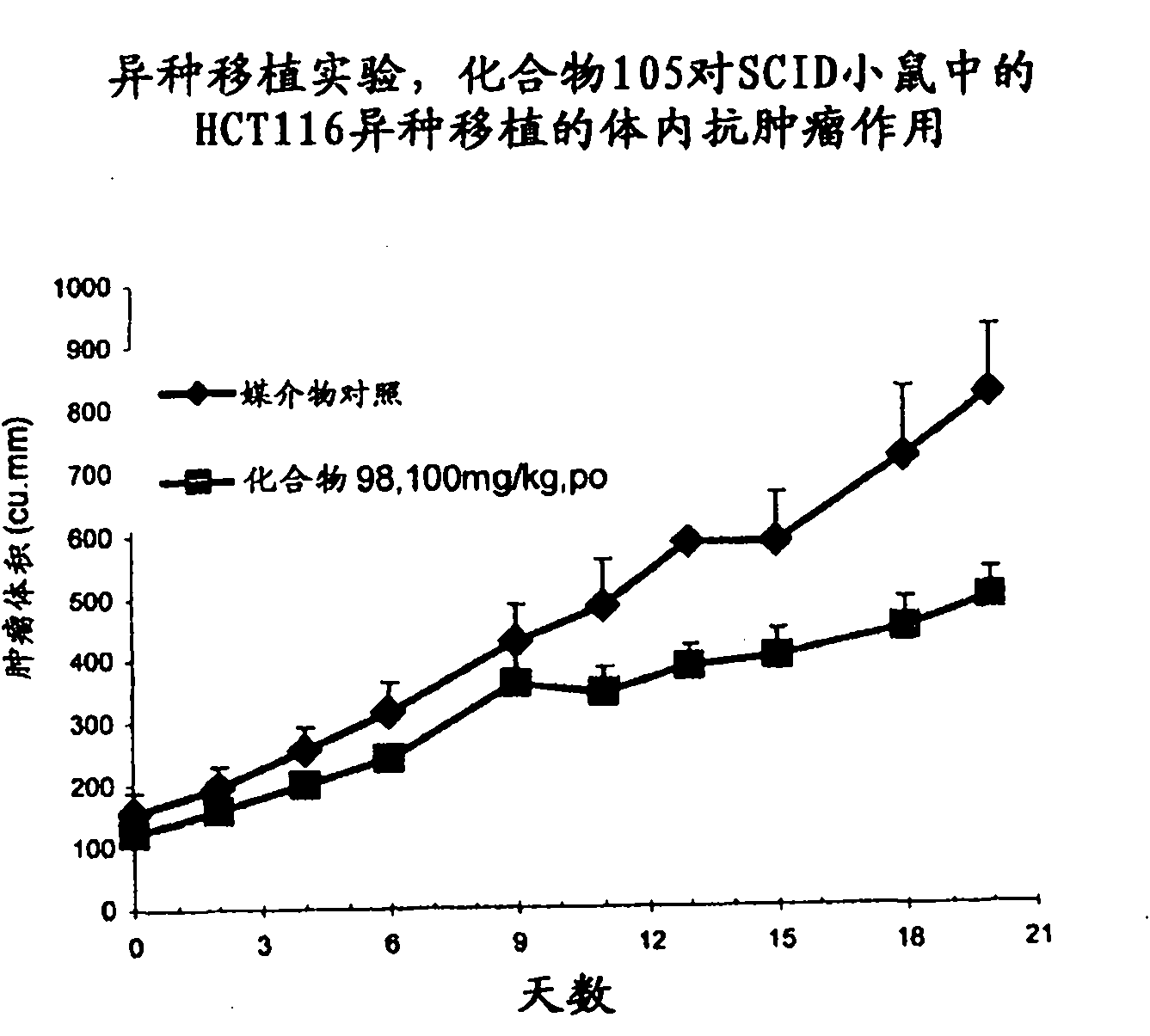
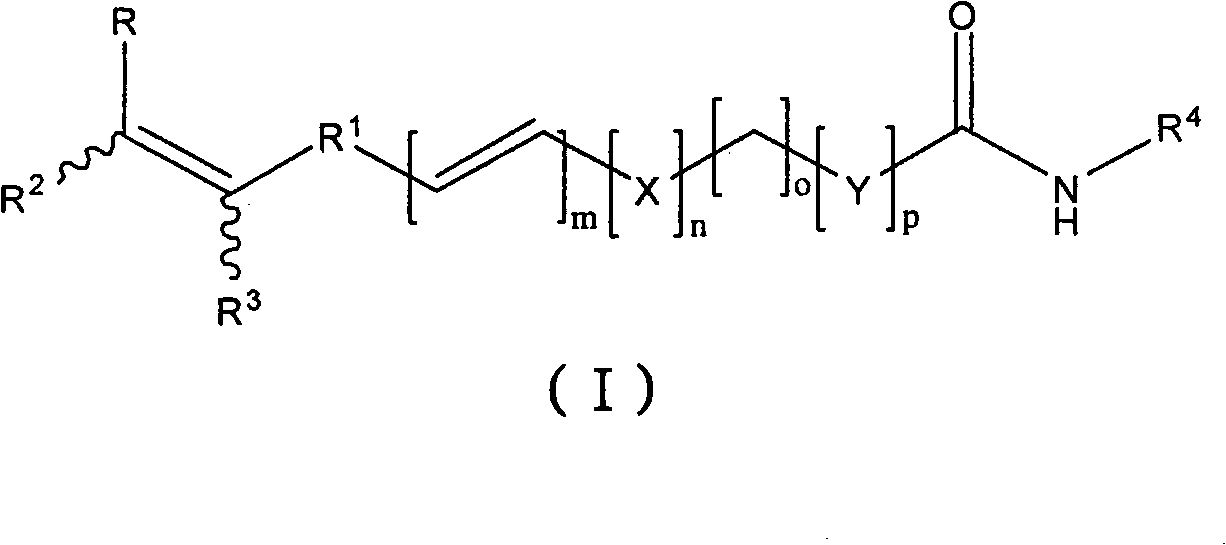
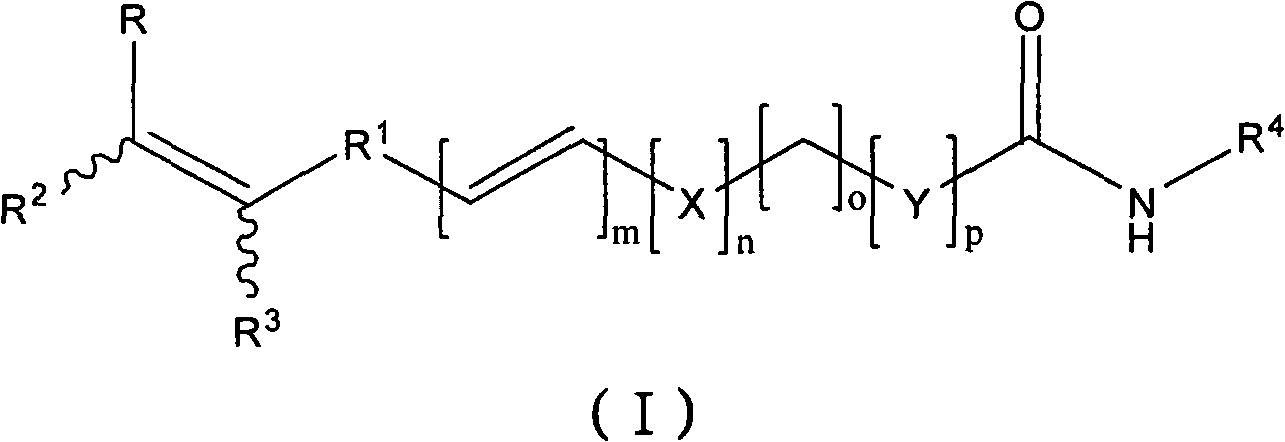
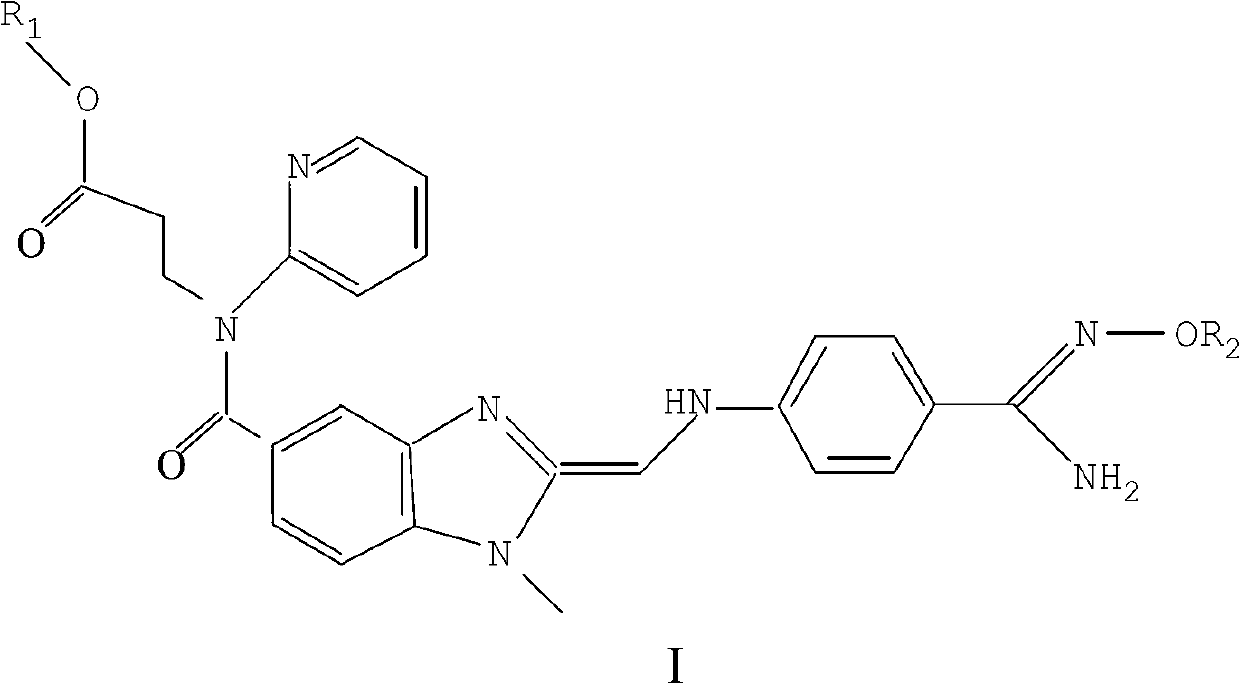
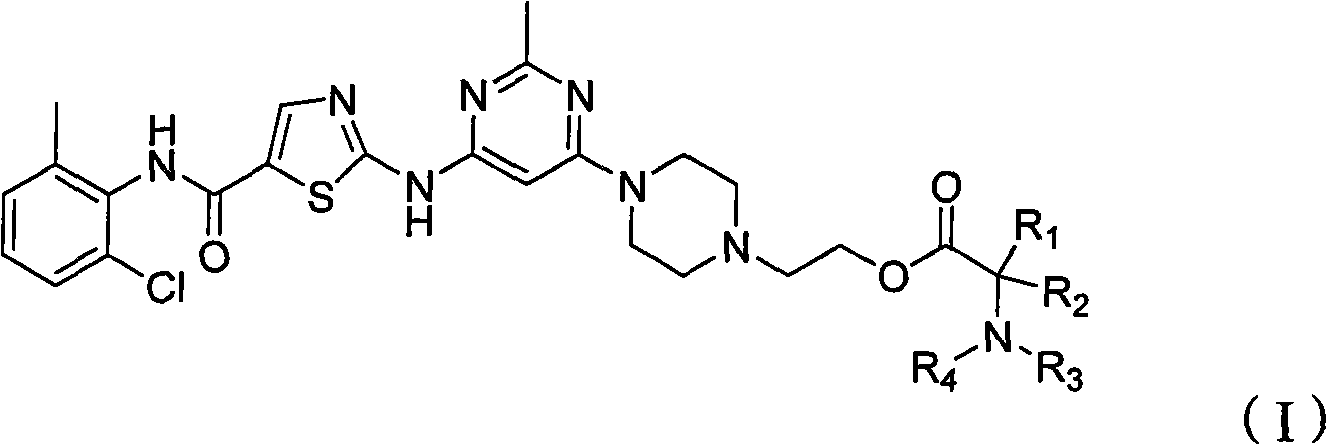
Antineoplastic compound, medicament composition and use thereof
The invention relates to an anti-tumor compound, the pharmaceutical composition thereof and the application thereof, particularly an anti-tumor compound shown as the formula (I) or pharmaceutically acceptable salt thereof, solvate thereof, polymorph thereof, enantiomer thereof or racemic mixture thereof; the pharmaceutical composition thereof, the application in the preparation of antitumor drugs, and the preparation method thereof. The compounds can be used as the prodrug of Dasatinib to produce Dasatinib by enzymolysis. The compounds interact with intestinal protein transporters (such as PEPT1 or PEPT2) so that the compounds can be easily absorbed in an intestinal tract in comparison with the Dasatinib to generate higher Dasatinib bioavailability.
Owner:BEIJING LABSOLUTIONS PHARMA
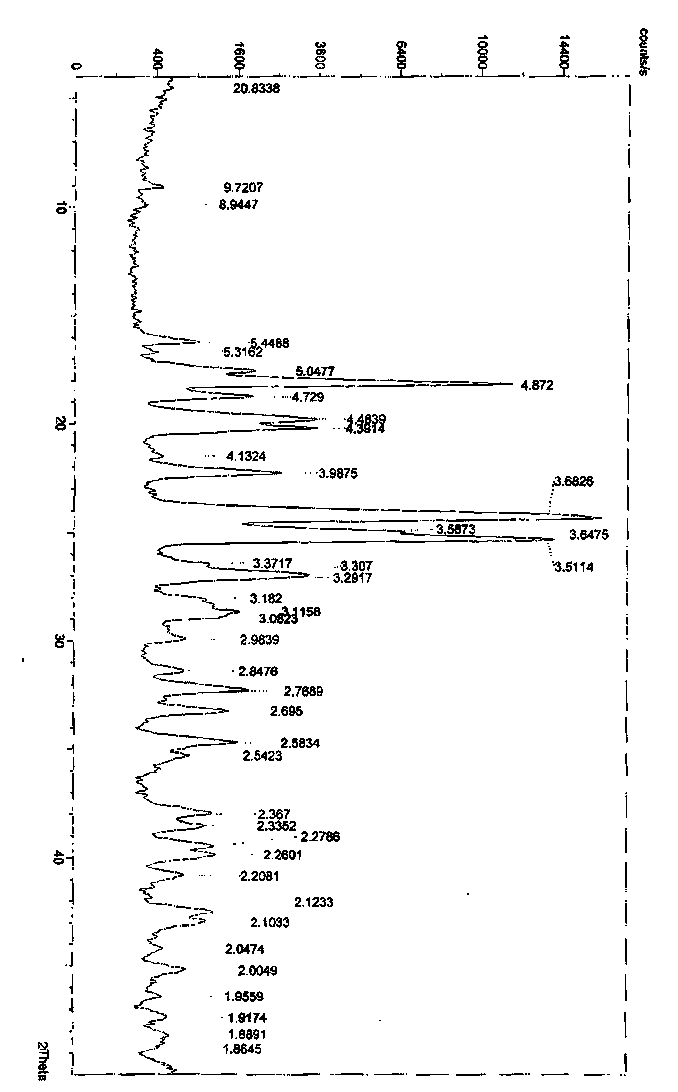
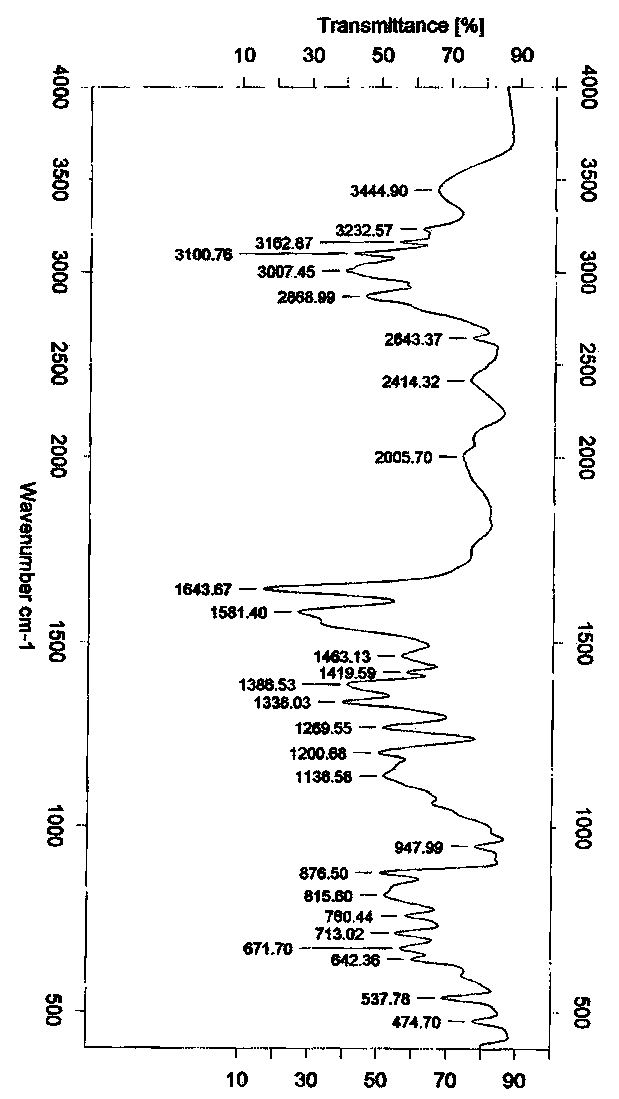
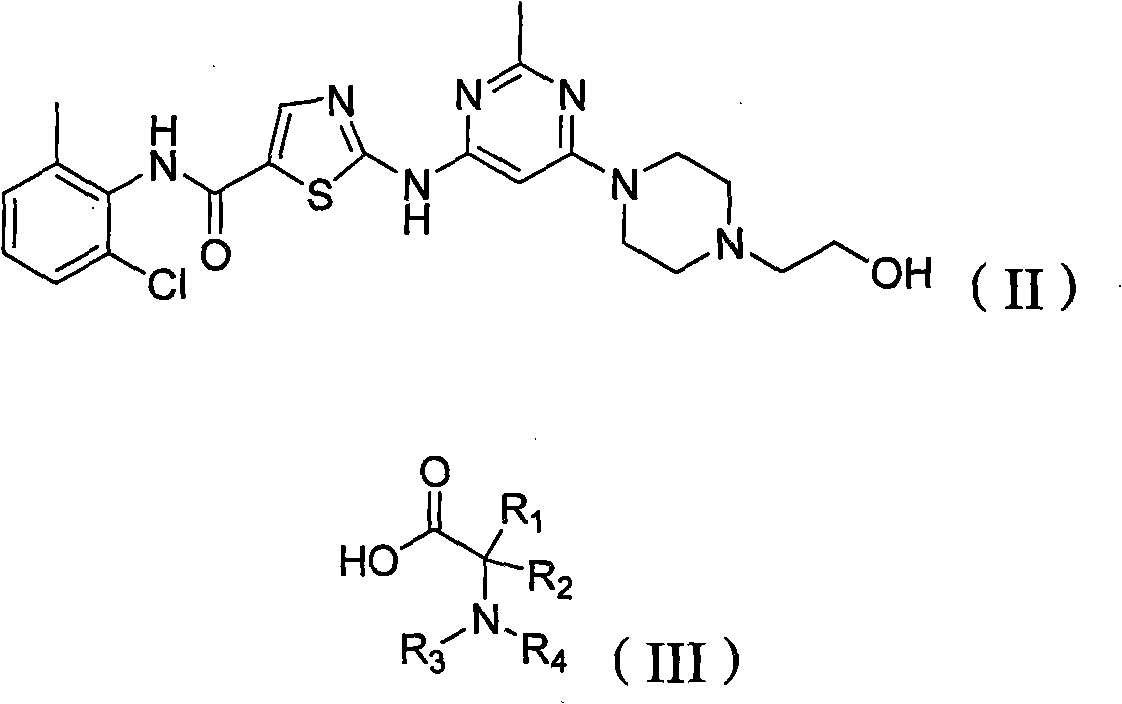
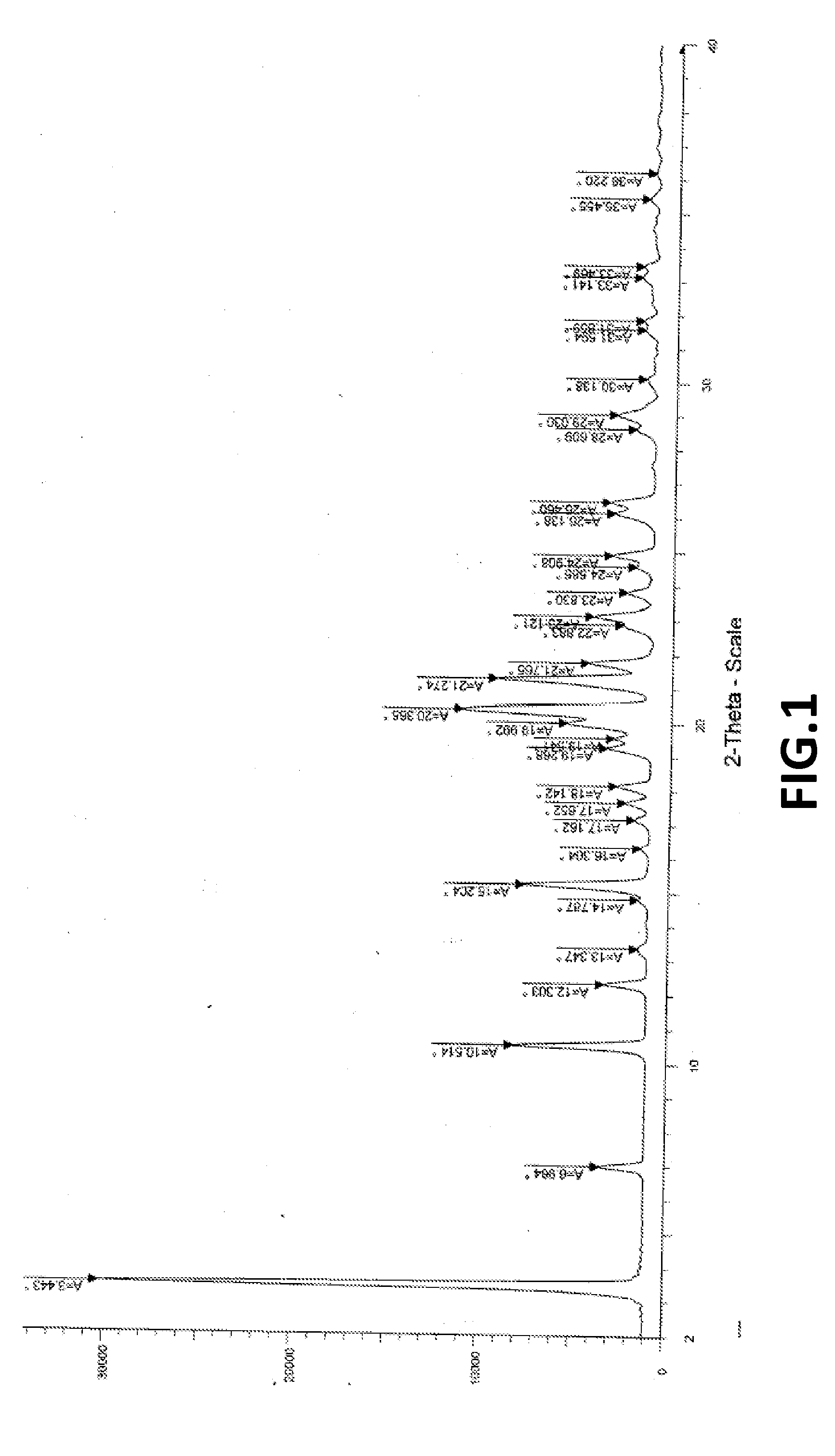
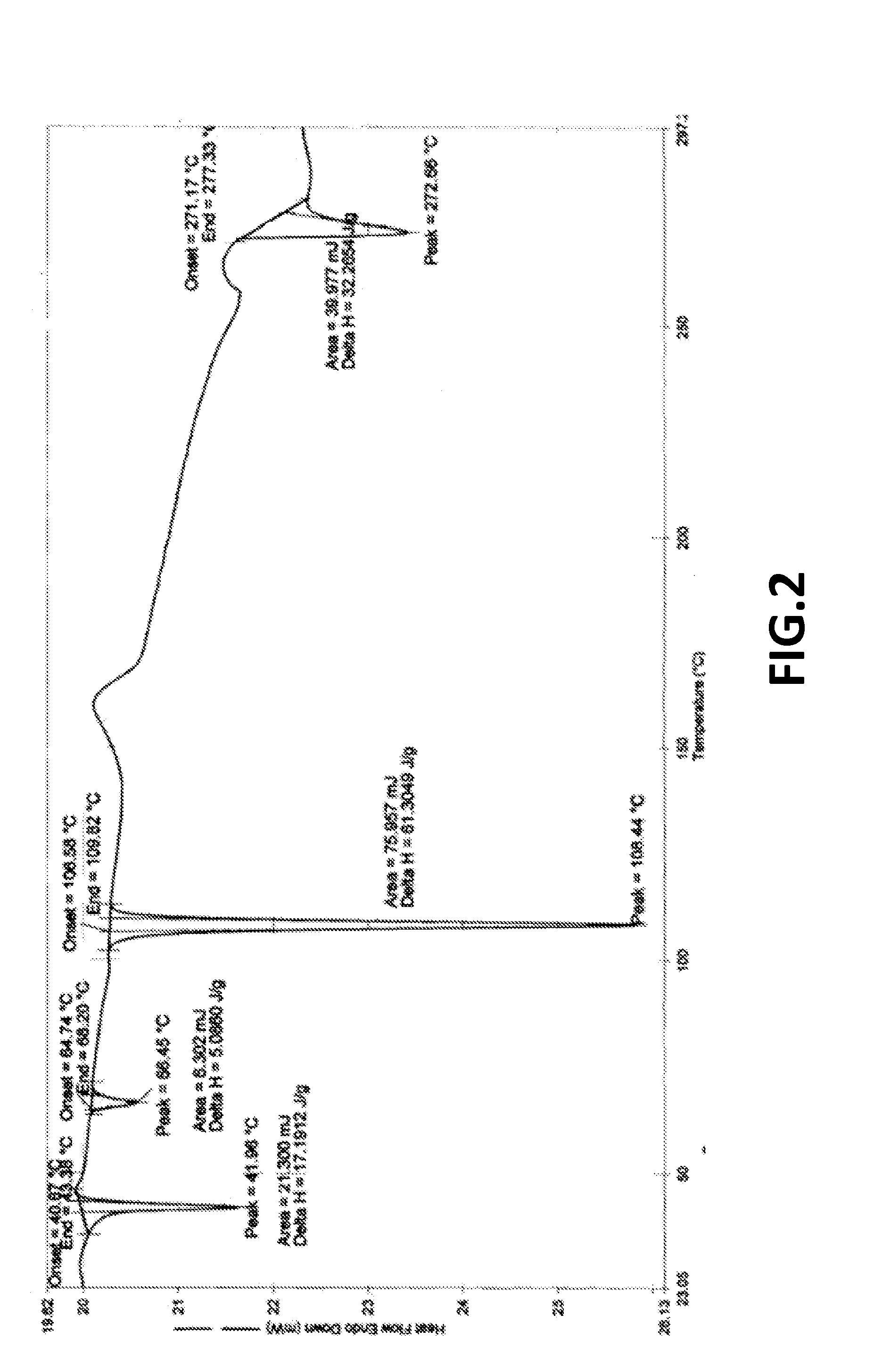
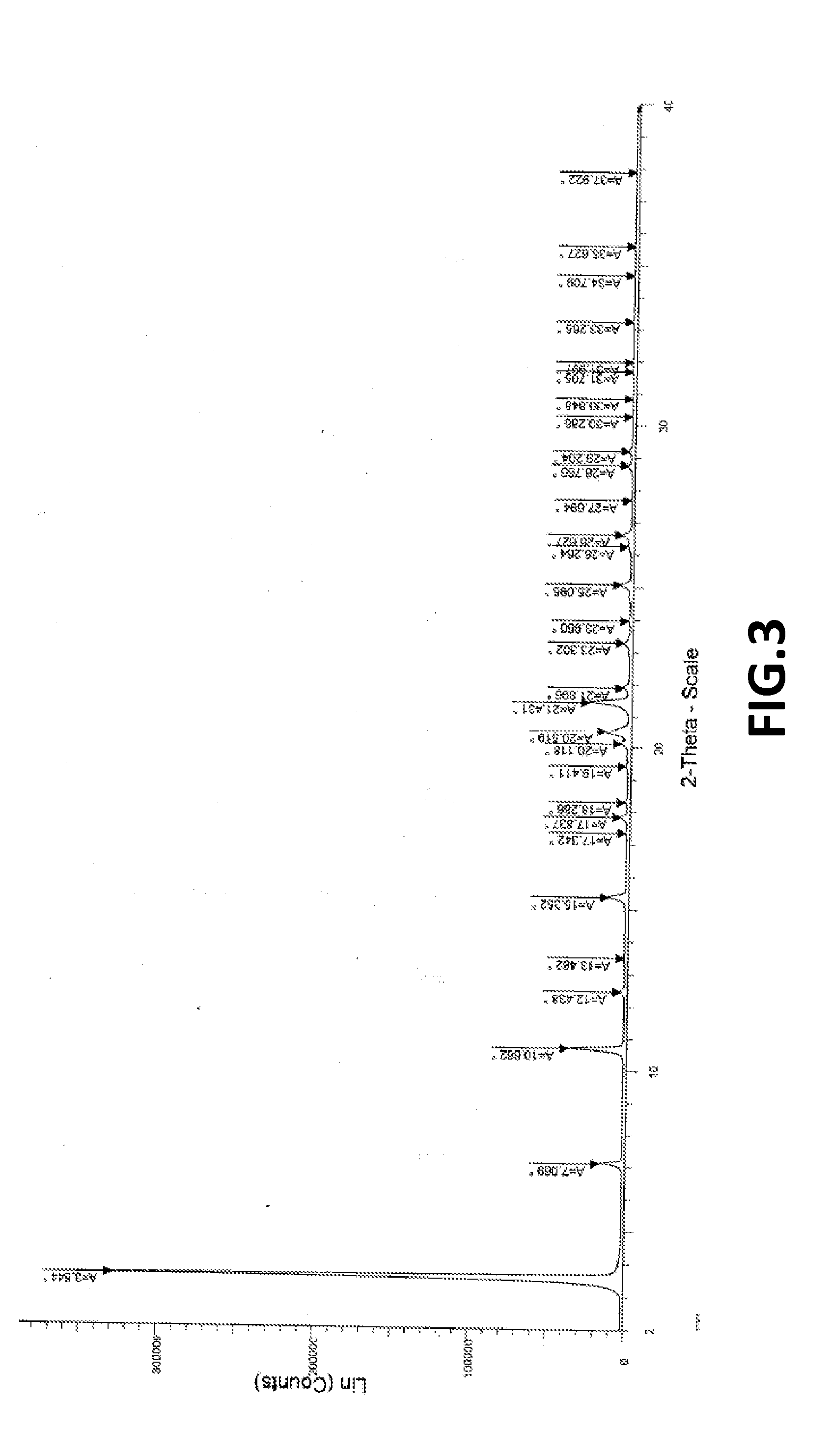
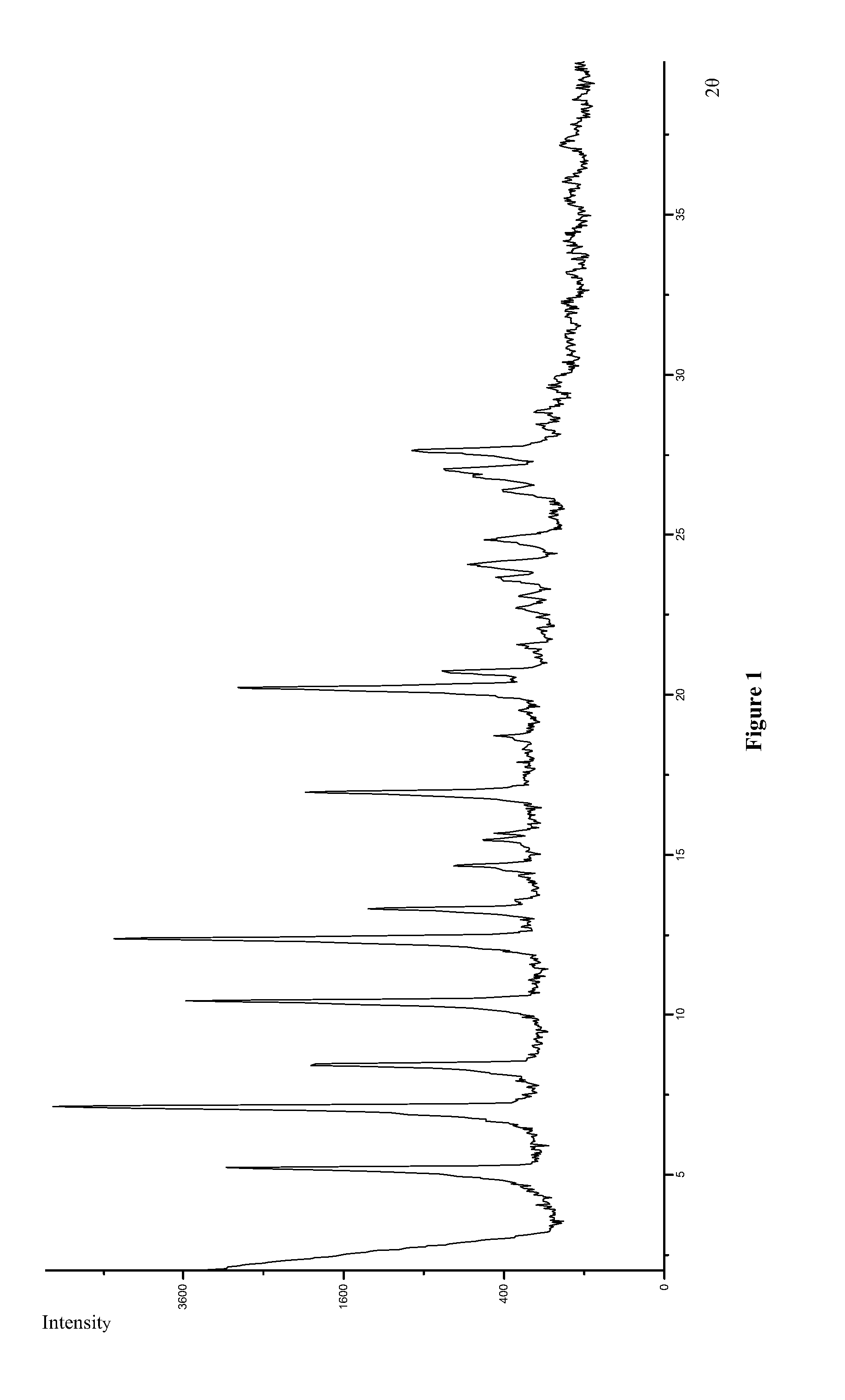
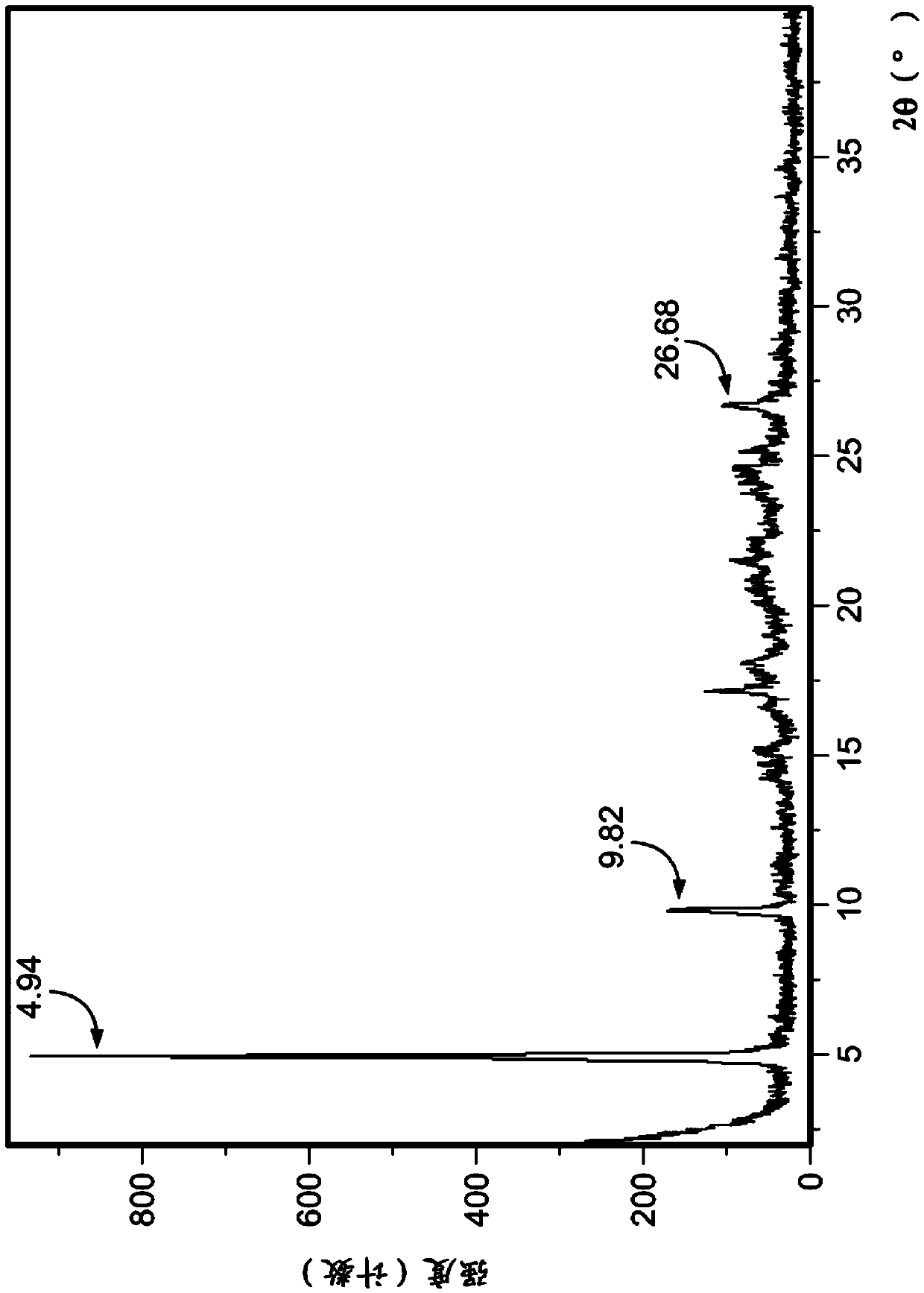
Crystalline form of pyrimidio[6,1-A]isoquinolin-4-one compound
The current invention is directed towards a polymorph of N-{2-[(2E)-2-(mesitylimino)-9,10-dimethoxy-4-oxo-6,7-dihydro-2H-pyrimido[6,1-a]-isoquinolin-3(4H)-yl]ethyl}urea, in the form of a crystalline solid consisting of greater than 99% by weight of N-{2-[(2E)-2-(mesitylimino)-9,10-dimethoxy-4-oxo-6,7-dihydro-2H-pyrimido[6,1-a]-isoquinoIin-3(4H)-yl]ethyl}urea, at least 95% in the polymorphic form of a thermodynamically stable polymorph (I) of N-{2-[(2E)-2-(mesitylimino)-9,10-dimetlioxy-4-oxo-6,7-dihydro-2H-pyrimido[6,1-a]-isoquinolin-3(4H)-yl]ethyl}urea, wherein said polymorph is determined by single crystal X-ray structural analysis and X-ray powder diffraction pattern.
Owner:VERONA PHARMA
Polymorph of rifaximin and process for the preparation thereof
The present invention relates to a new polymorph of Rifaximin, designated κ, and to a process for the preparation thereof. Under certain aspects, the invention also relates to pharmaceutical compositions comprising an effective amount of the polymorphic form κ of Rifaximin and a pharmaceutically acceptable carrier and its uses in the treatment of gastrointestinal conditions.
Owner:CLAROCHEM IRELAND
Fingolimod polymorphs and their processes
ActiveUS20130281739A1Organic compound preparationOrganic chemistry methodsRelated impuritiesMedicinal chemistry
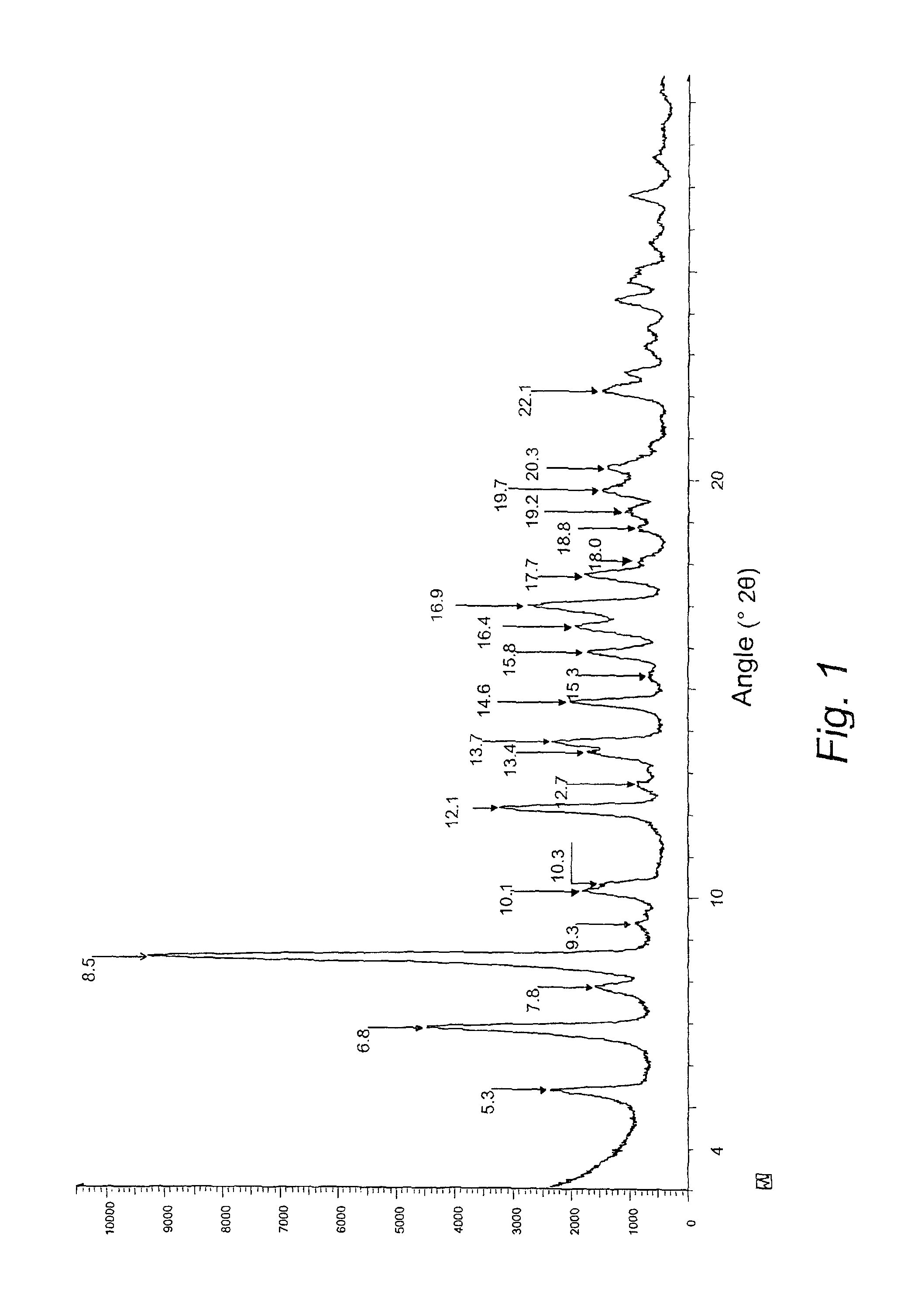
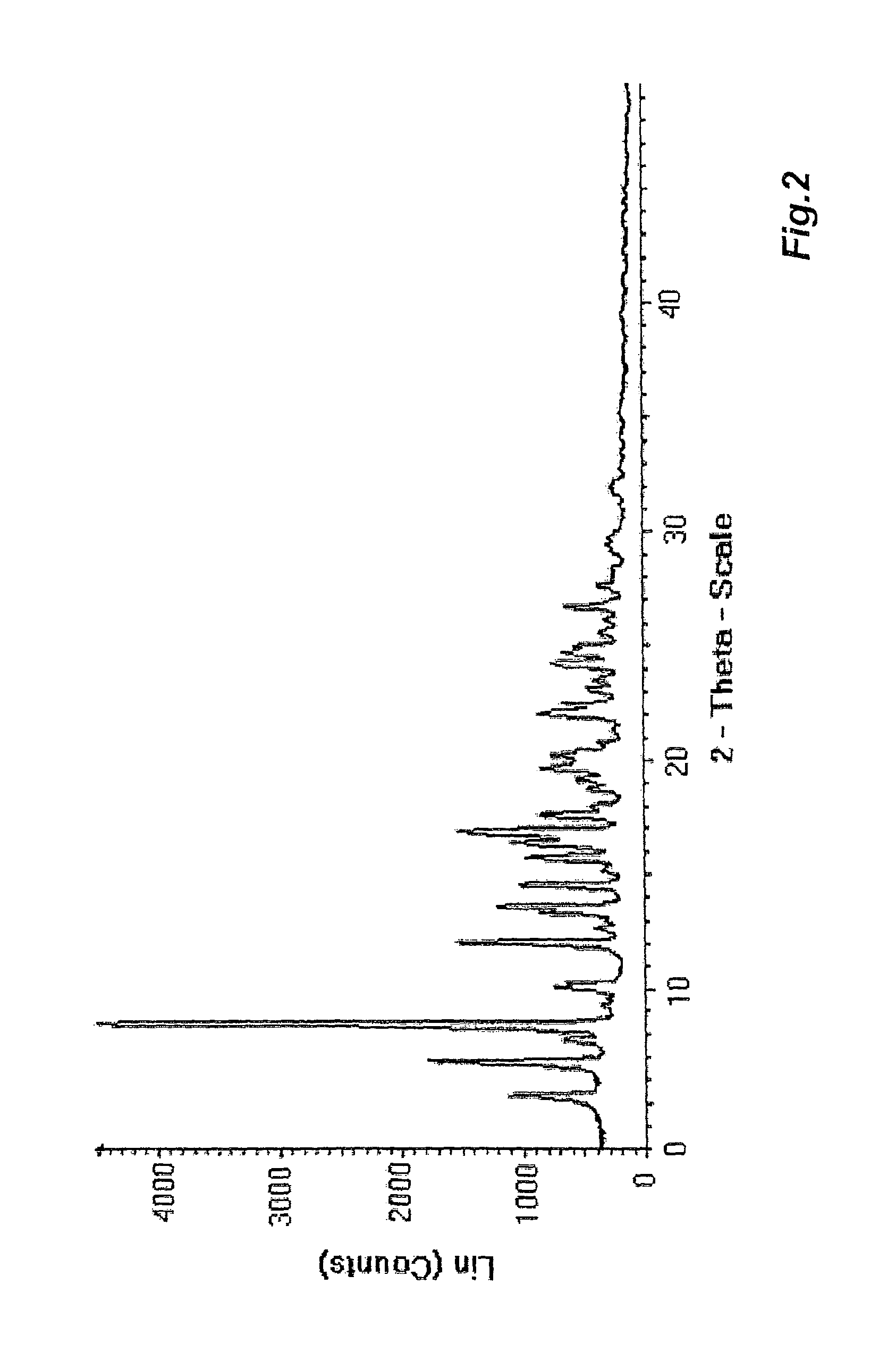
The present invention provides crystalline polymorphic forms of Fingolimod HCl (I) and processes for preparation thereof.The application provides processes for preparation of crystalline polymorphic forms-α, β and μ substantially free from process related impurities. The crystalline polymorphic forms of Fingolimod HCl (I) obtained by the processes according to the present invention having an XRDP pattern as per FIGS. 1, 3 and 5, which are useful as active pharmaceutical ingredient in pharmaceutical compositions for the treatment or prevention of autoimmune related disorder including multiple sclerosis.
Owner:SHILPA PHARM INC
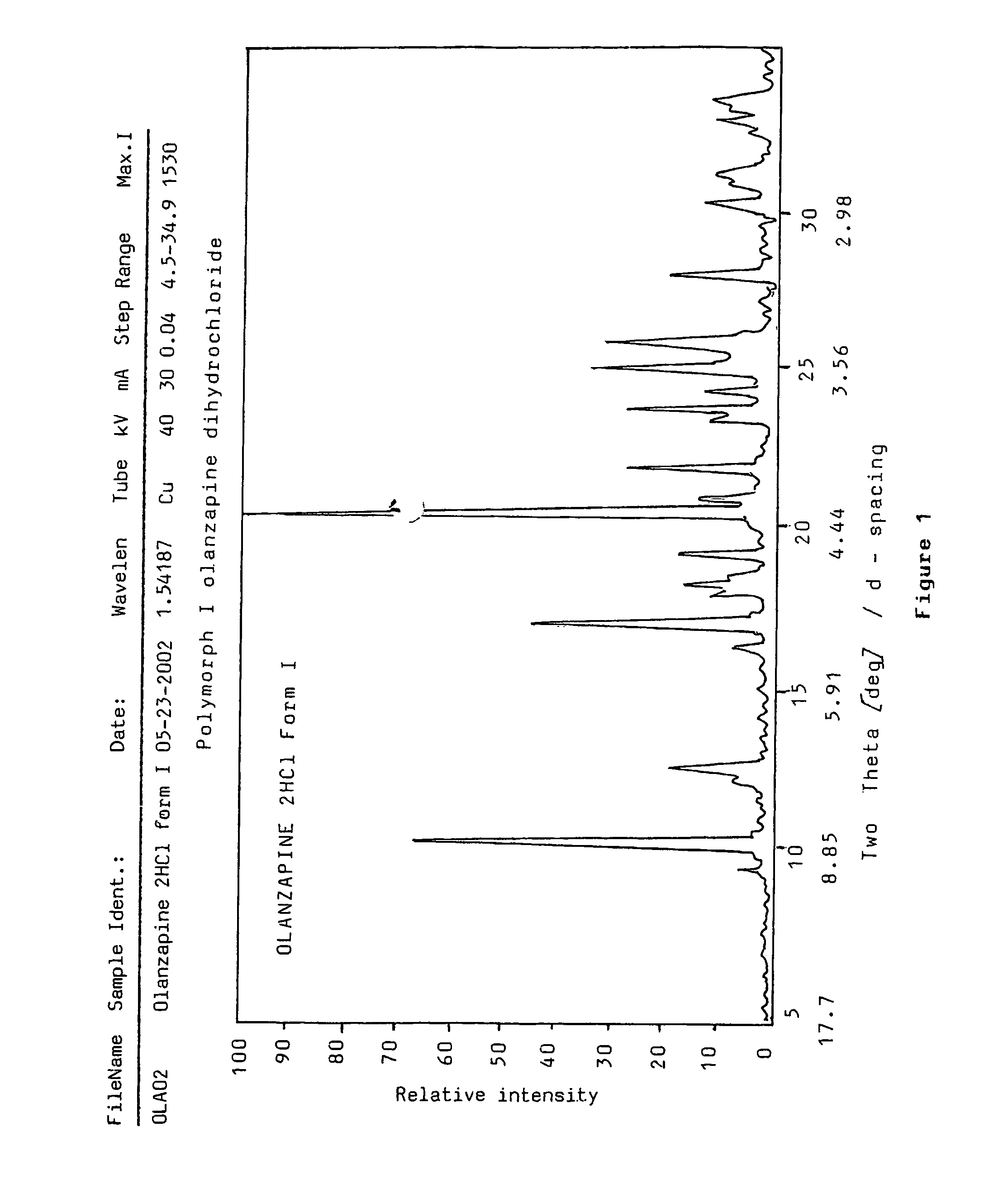
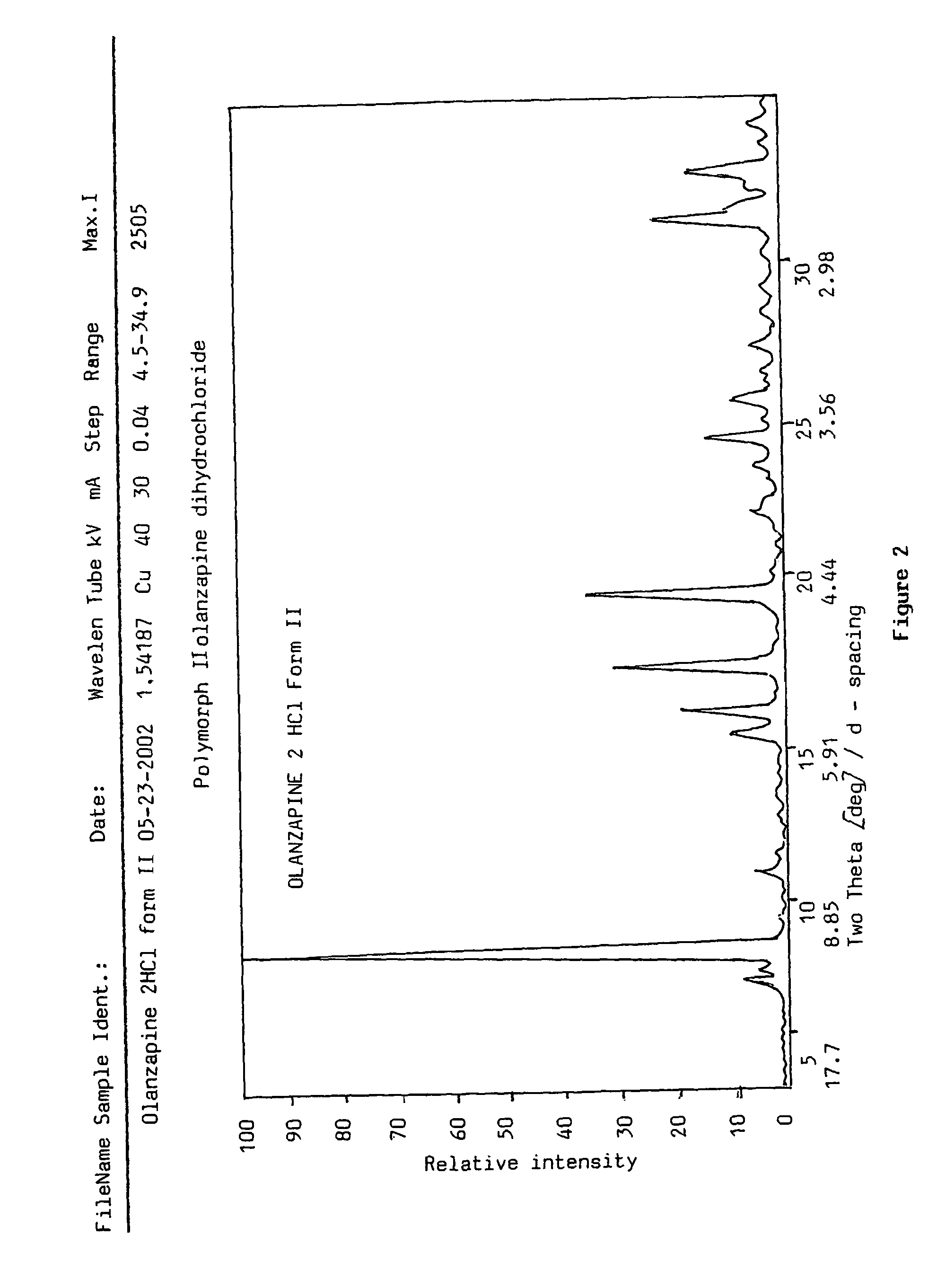
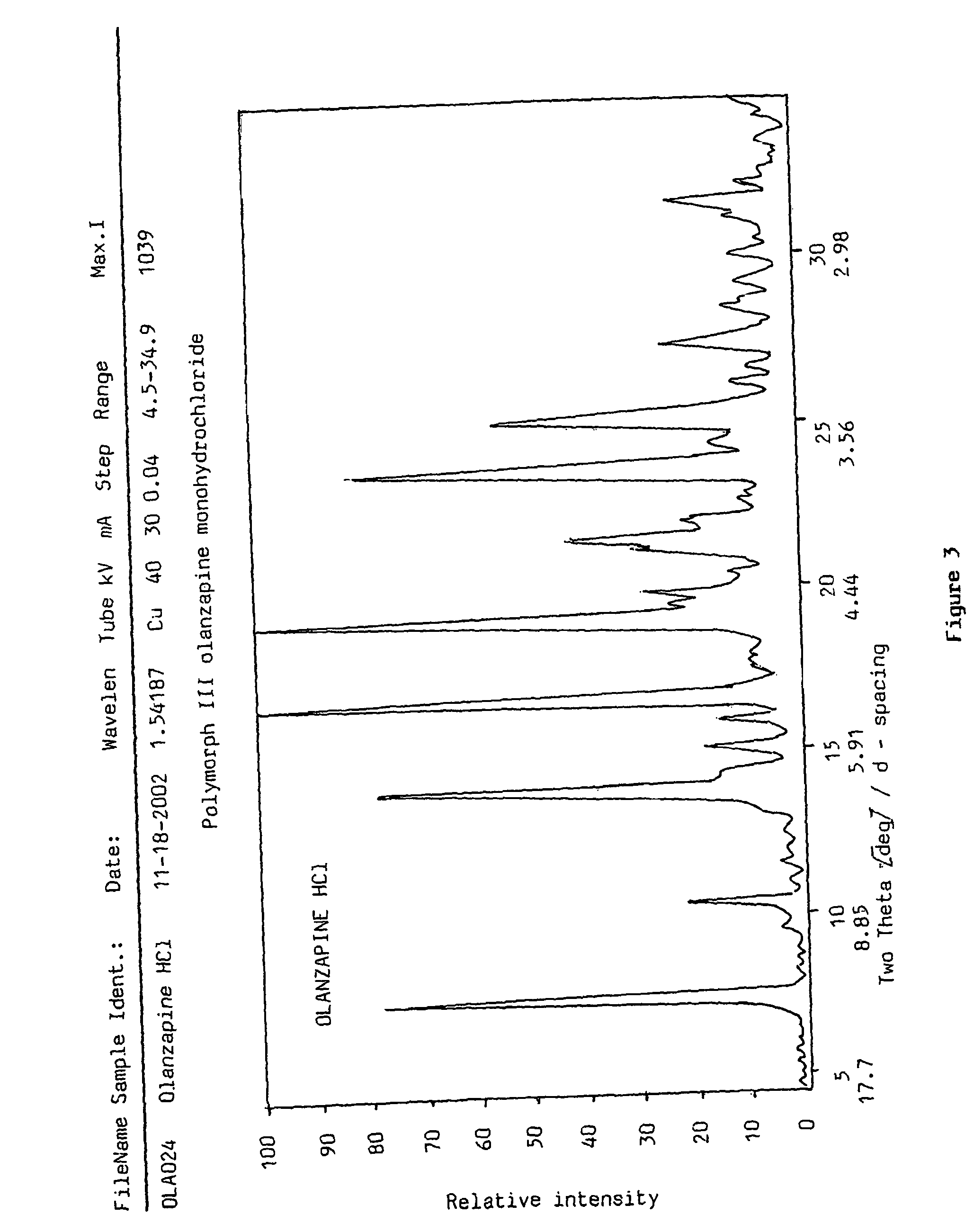
Polymorphs of olanzapine hydrochloride
InactiveUS7951798B2Constant filtration and drying characteristicHigh purityBiocideNervous disorderBenzodiazepineMethyl group
The present invention relates to new crystalline forms I, II and III of 2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3-b][1,5]-benzodiazepine hydrochloride, a process for the preparation thereof and pharmaceutical compositions containing the same. Said new polymorphic forms are useful as active ingredients for the treatment of psychotic conditions.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENY TARSASAG
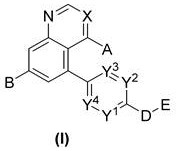
New compound used as rearranged during transfection kinase inhibitor
The present invention relates to a compound, a pharmaceutical composition containing the compound, a preparation method of the compounds, and application of the same as a rearranged during transfection (RET) kinase inhibitor. The compound is a compound shown as formula (I), or a pharmaceutically acceptable salt, a prodrug, a solvent compound, a polymorph, an isomer and a stable isotope derivativethereof. The present invention also relates to the application of the compounds to treatment or prevention of RET kinase mediated related diseases like tumors and a method of using the compounds for the treatment of the diseases.
Owner:NANJING INNOCARE PHARMA TECH CO LTD
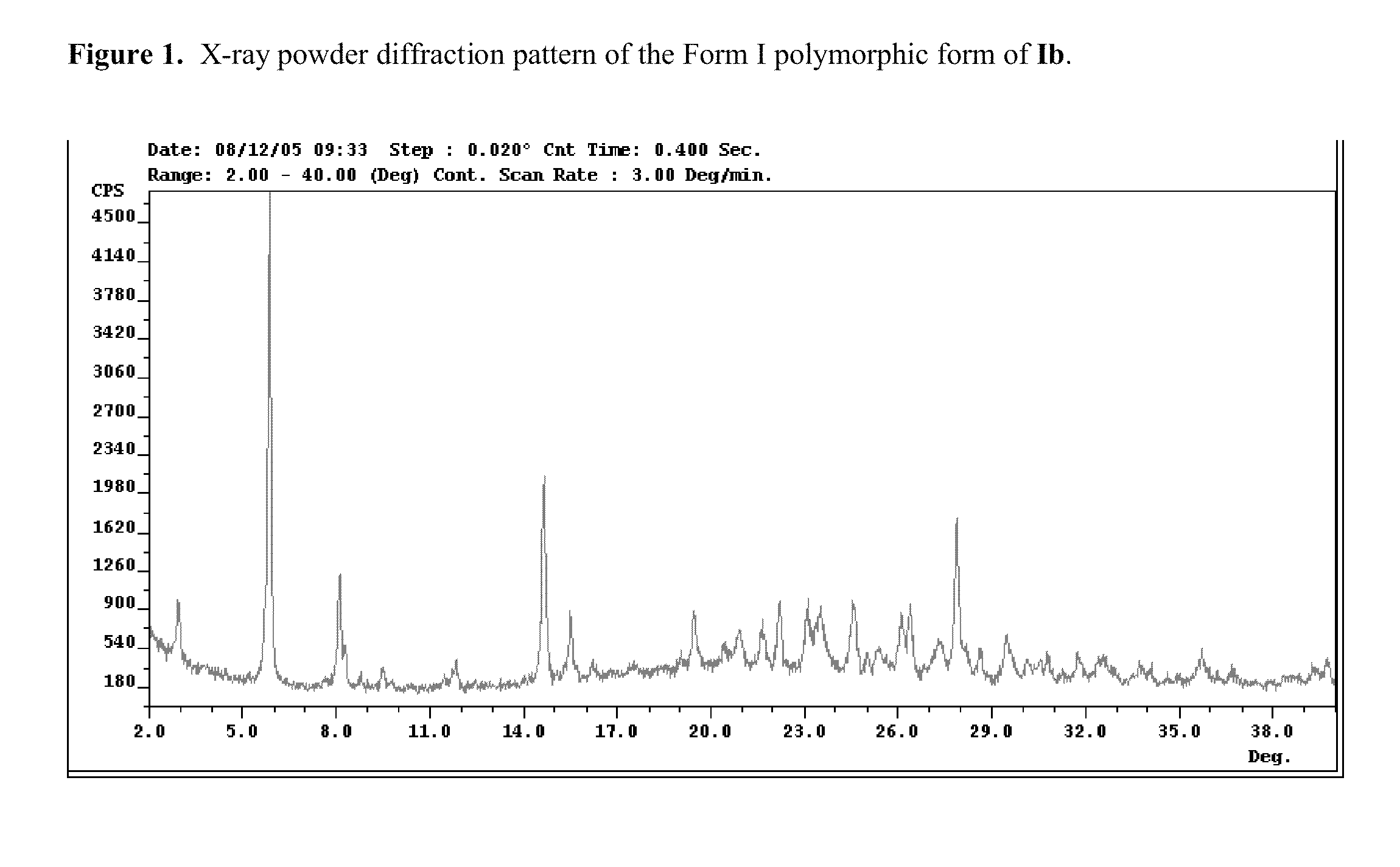
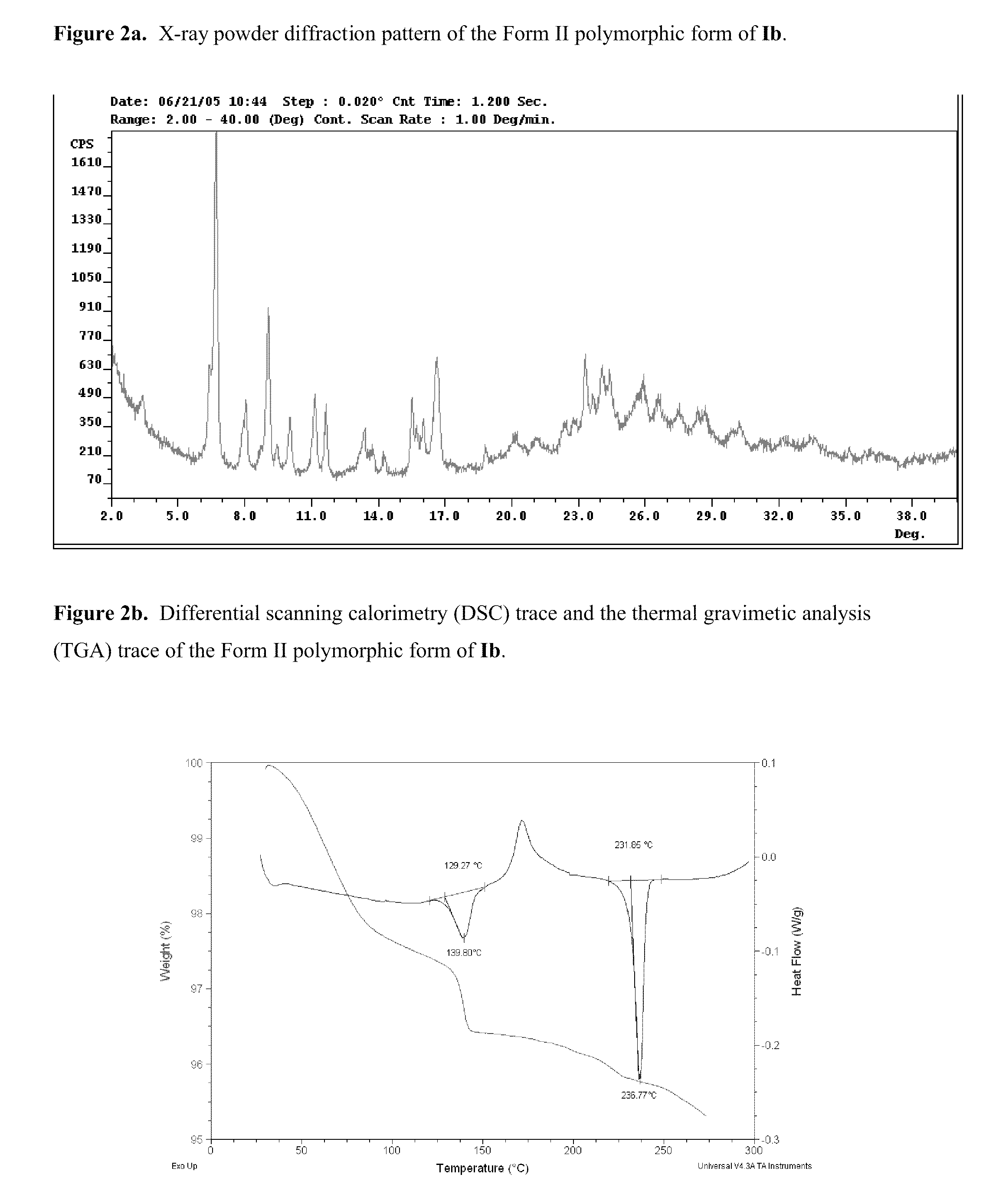
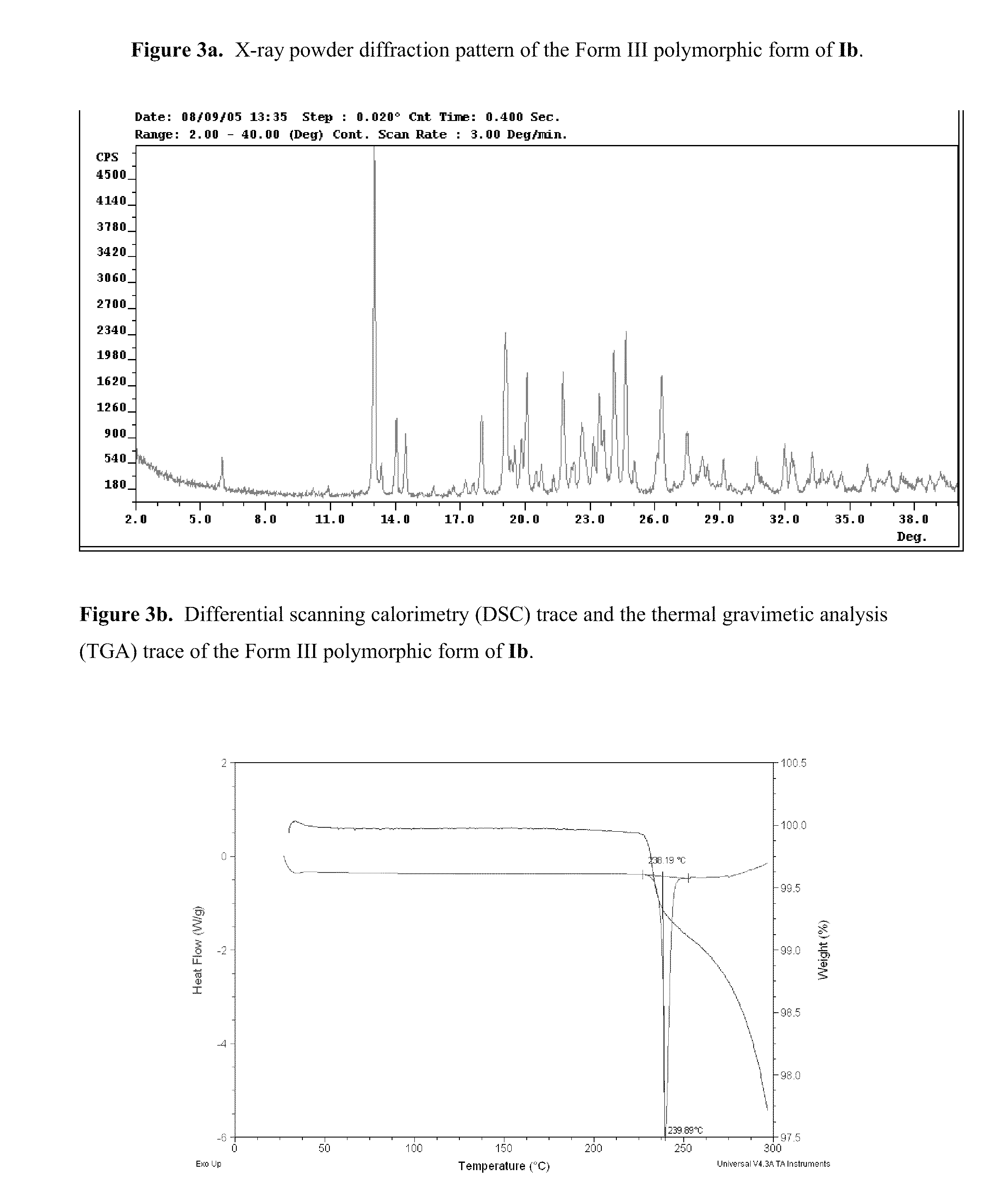
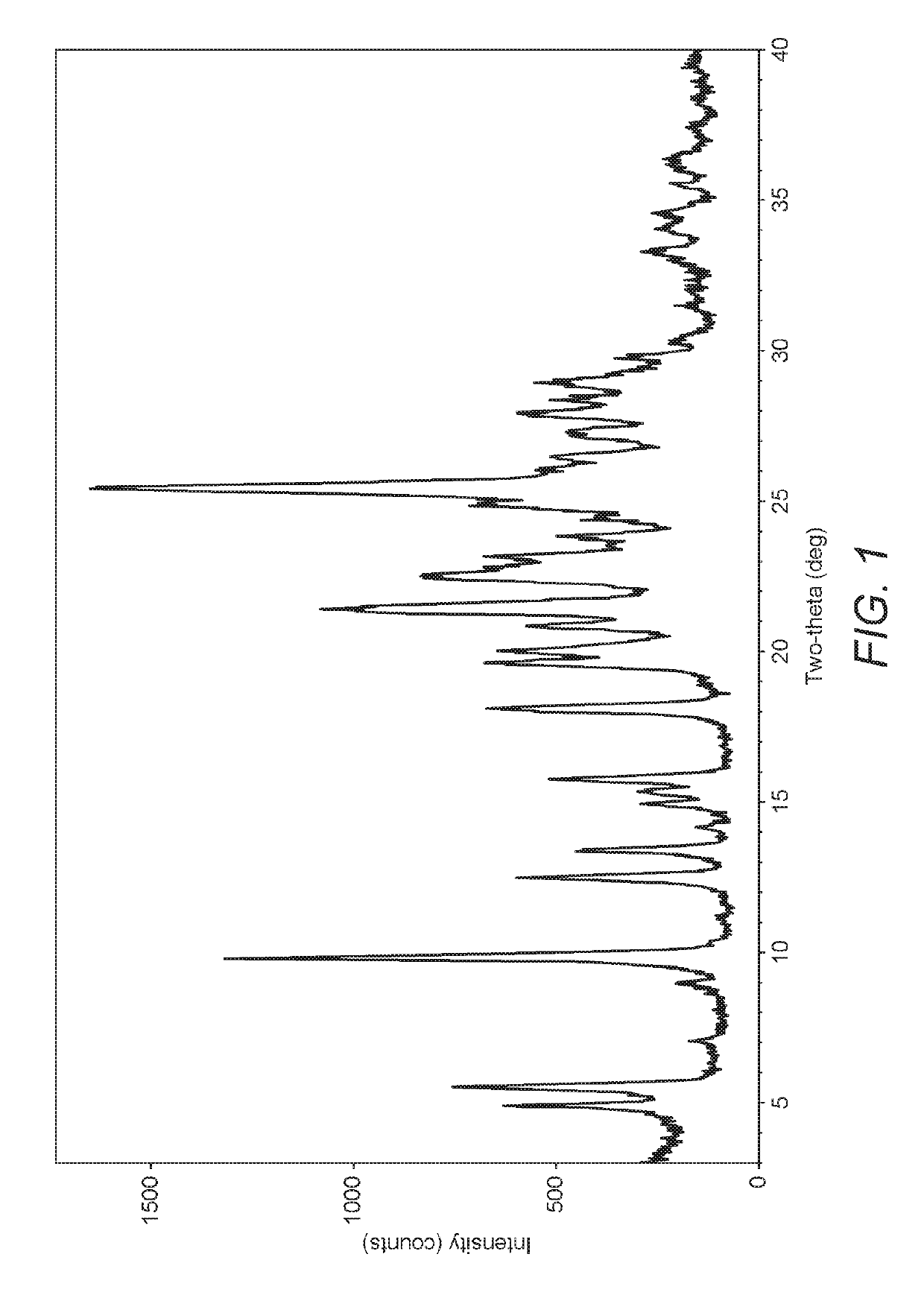
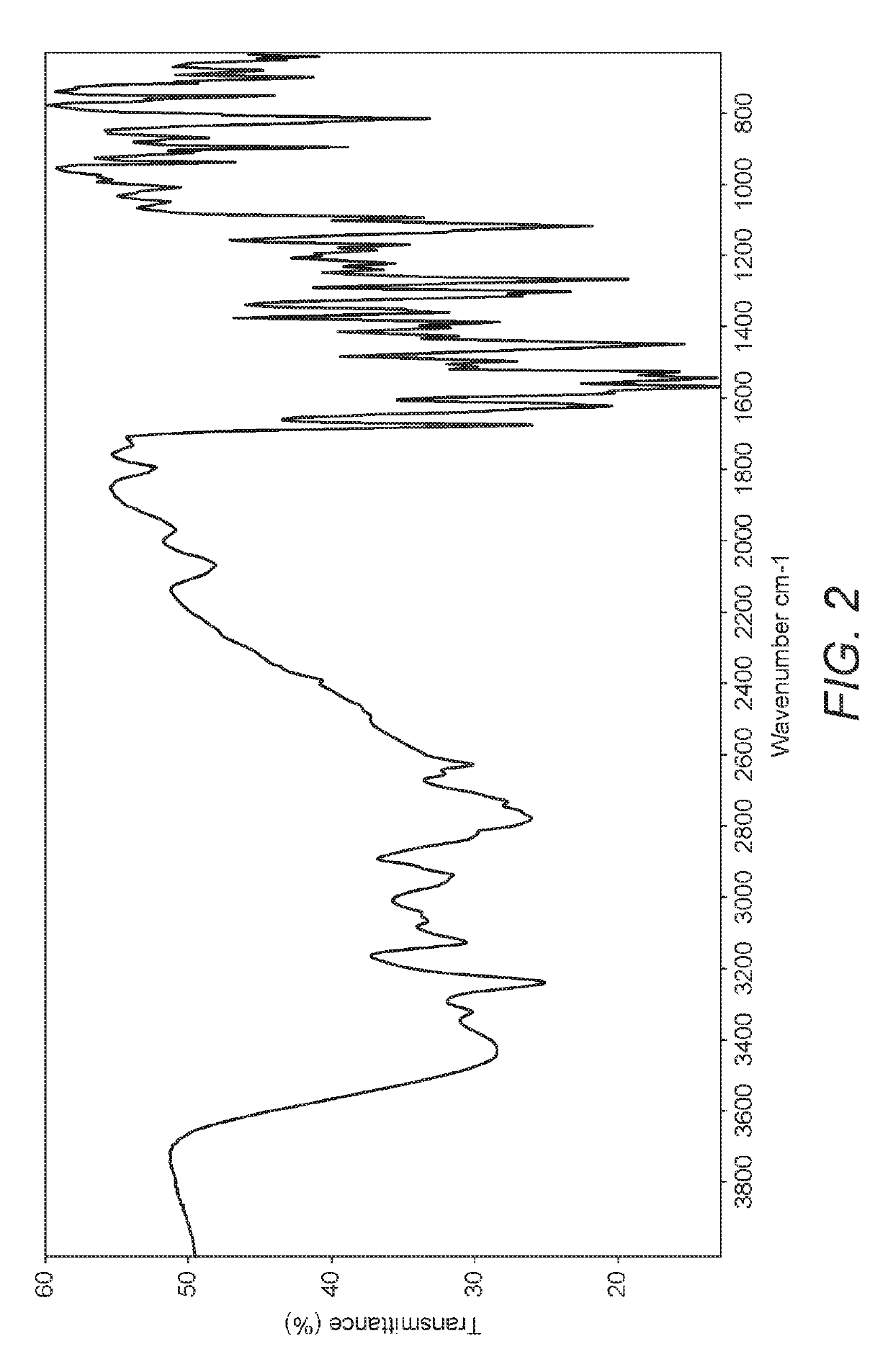
Polymorphs of acyl sulfonamides
The application discloses novel polymorphic crystalline forms of 2-[4-Bromo-3-(3-chloro-5-cyano-phenoxy)-2-fluoro-phenyl]-N-(2-chloro-4-propionylsulfamoyl-phenyl)-acetamide, sodium salt (Ib)with improved stability and physical properties which facilitate manufacturing, handling and formulating for treatment or prophylaxis of HIV mediated diseases, AIDS or ARC, in monotherapy or in combination therapy.
Owner:ROCHE PALO ALTO LLC
Polymorphic form X of nilotinib dihydrochloride hydrate
InactiveUS10301282B2Improve permeabilityImprove solubilityOrganic chemistry methodsAntineoplastic agentsMedicinal chemistryNILOTINIB HYDROCHLORIDE
The present invention relates to a novel polymorph of nilotinib hydrochloride (Form X), to processes for its preparation, to pharmaceutical compositions containing the same and to its use in medicine.
Owner:CIPLA LTD
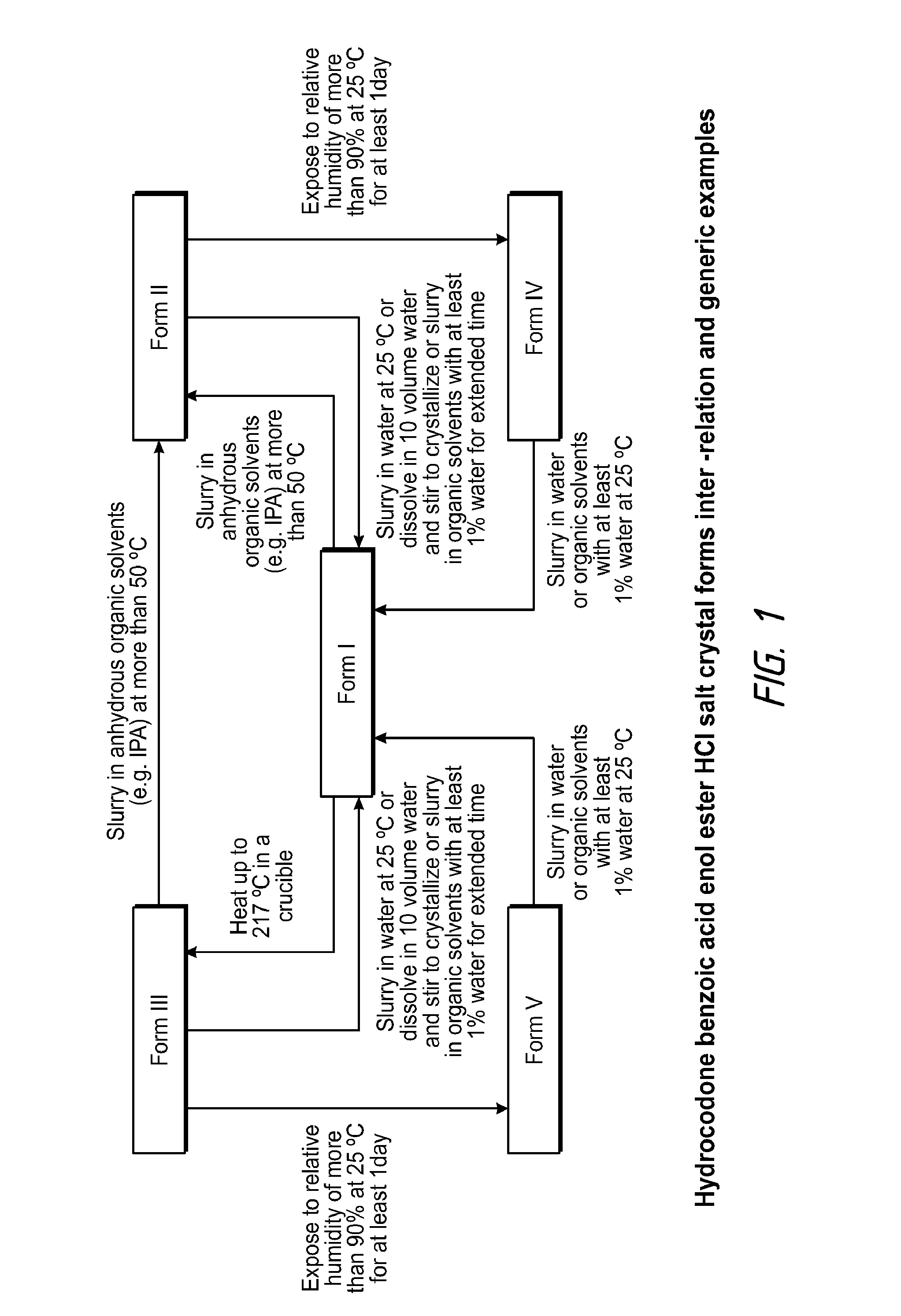
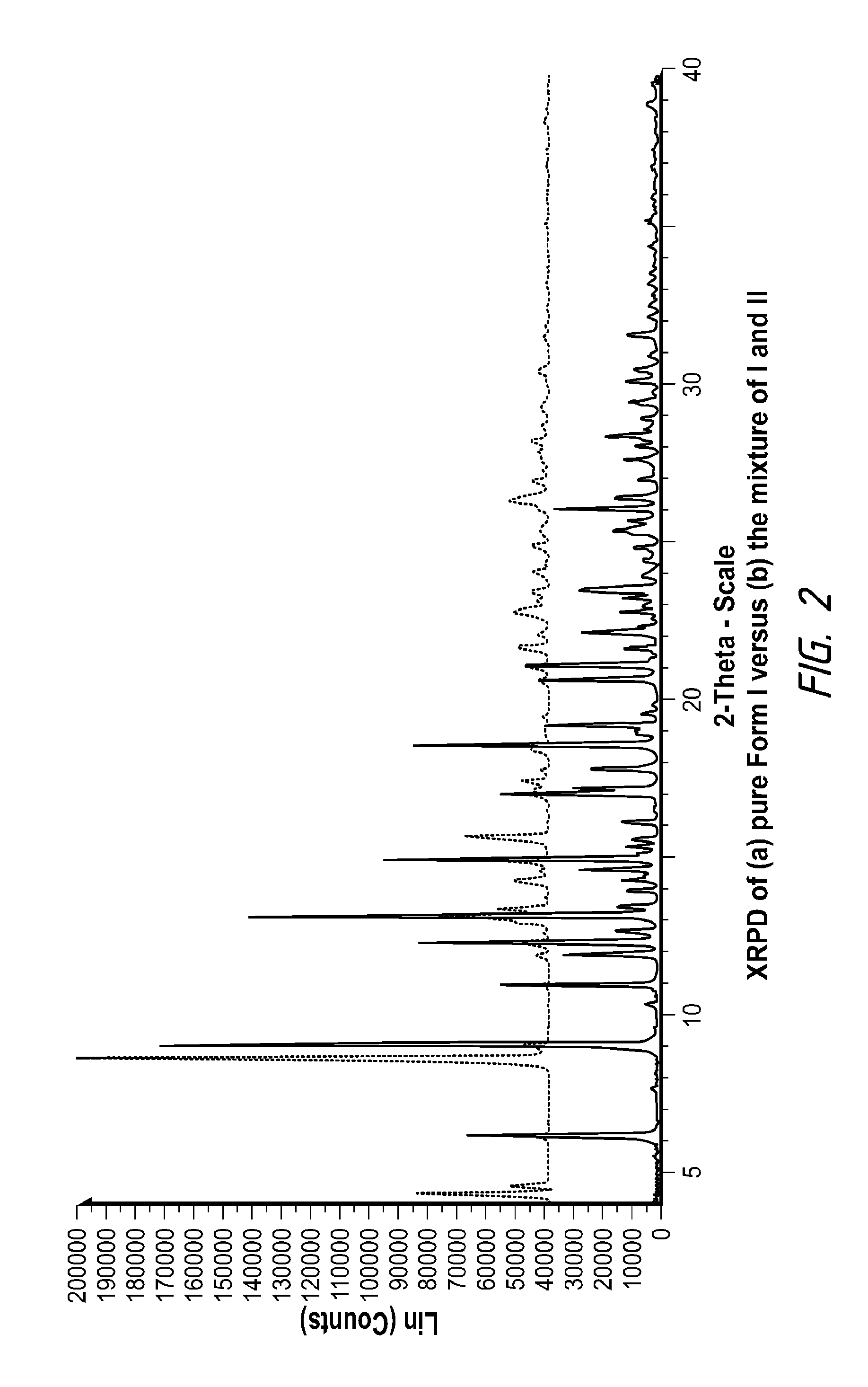
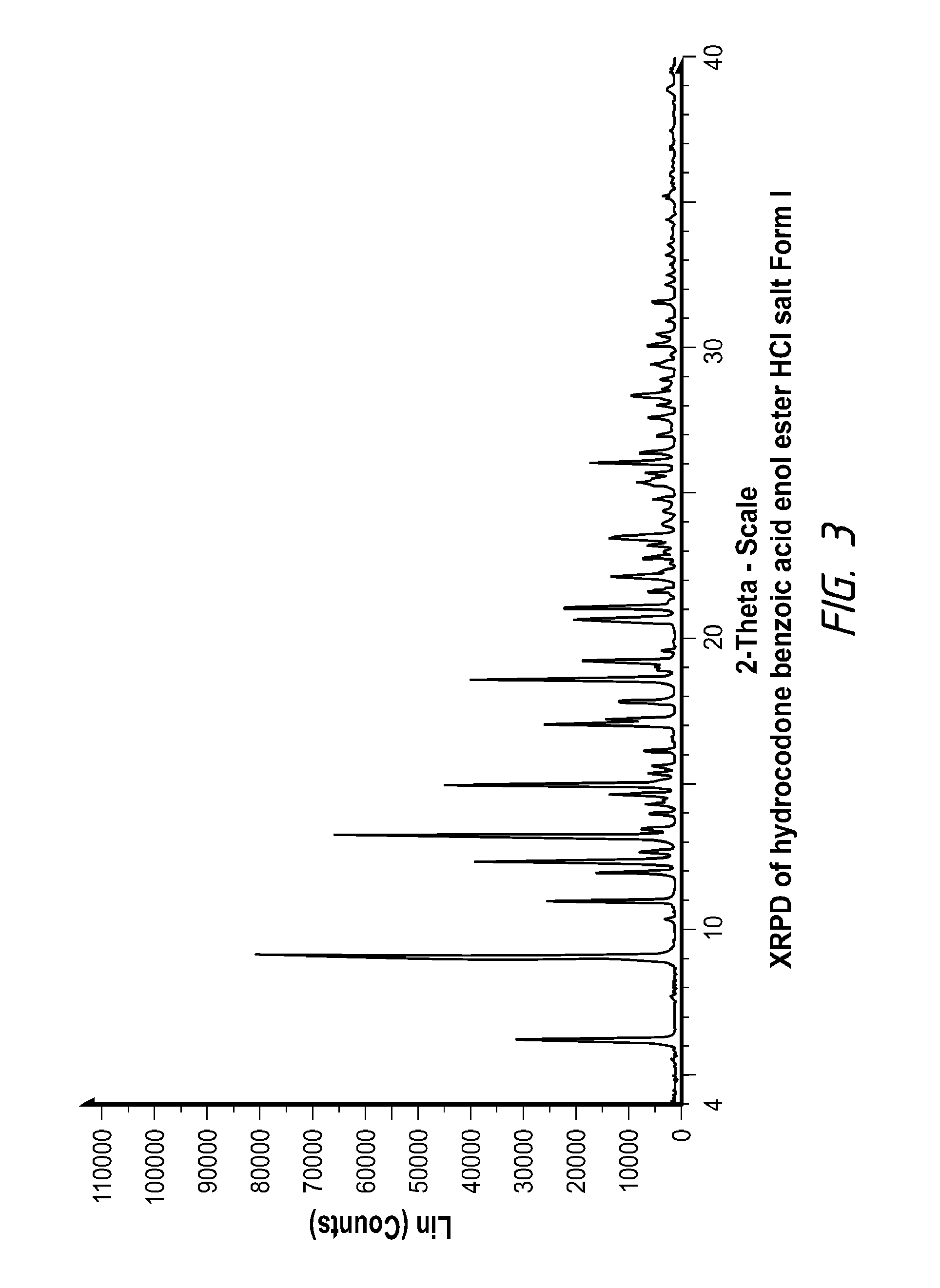
Pharmaceutically Acceptable Salts and Polymorphic Forms of Hydrocodone Benzoic Acid Enol Ester and Processes for Making Same
Compositions comprising hydrocodone benzoic acid enol ester to form novel prodrugs including hydrocodone benzoic acid enol ester salts, and various polymorphs. Also provided are processes for the preparation of hydrocodone benzoic acid enol ester salts, and various polymorphs.
Owner:KEMPHARM INC +1
Compounds, compositions and methods
ActiveUS10202363B2Suitable for bulk preparation and handlingMore thermodynamically stableOrganic active ingredientsSenses disorderMedicineChemical compound
The disclosed subject matter provides certain polymorphic forms of Compound (I) as well as pharmaceutical compositions comprising Compound (I) or such polymorphic forms, and methods of using or making such compounds and pharmaceutical compositions. It has now been discovered that Compound (I) can exist in multiple crystalline forms (polymorphs). One particular crystalline form, Form II, has been found to be more thermodynamically stable and, thus, likely more suitable for bulk preparation and handling than other polymorphic forms. Efficient and economic methods have been developed to prepare Compound (I) and Form II in high purity on a large scale. In animal studies, Form II has demonstrated safety and efficacy in treating depressive disorders and, when micronized, improved absorption compared to non-micronized Form II.
Owner:MERCK SHARP & DOHME CORP +1
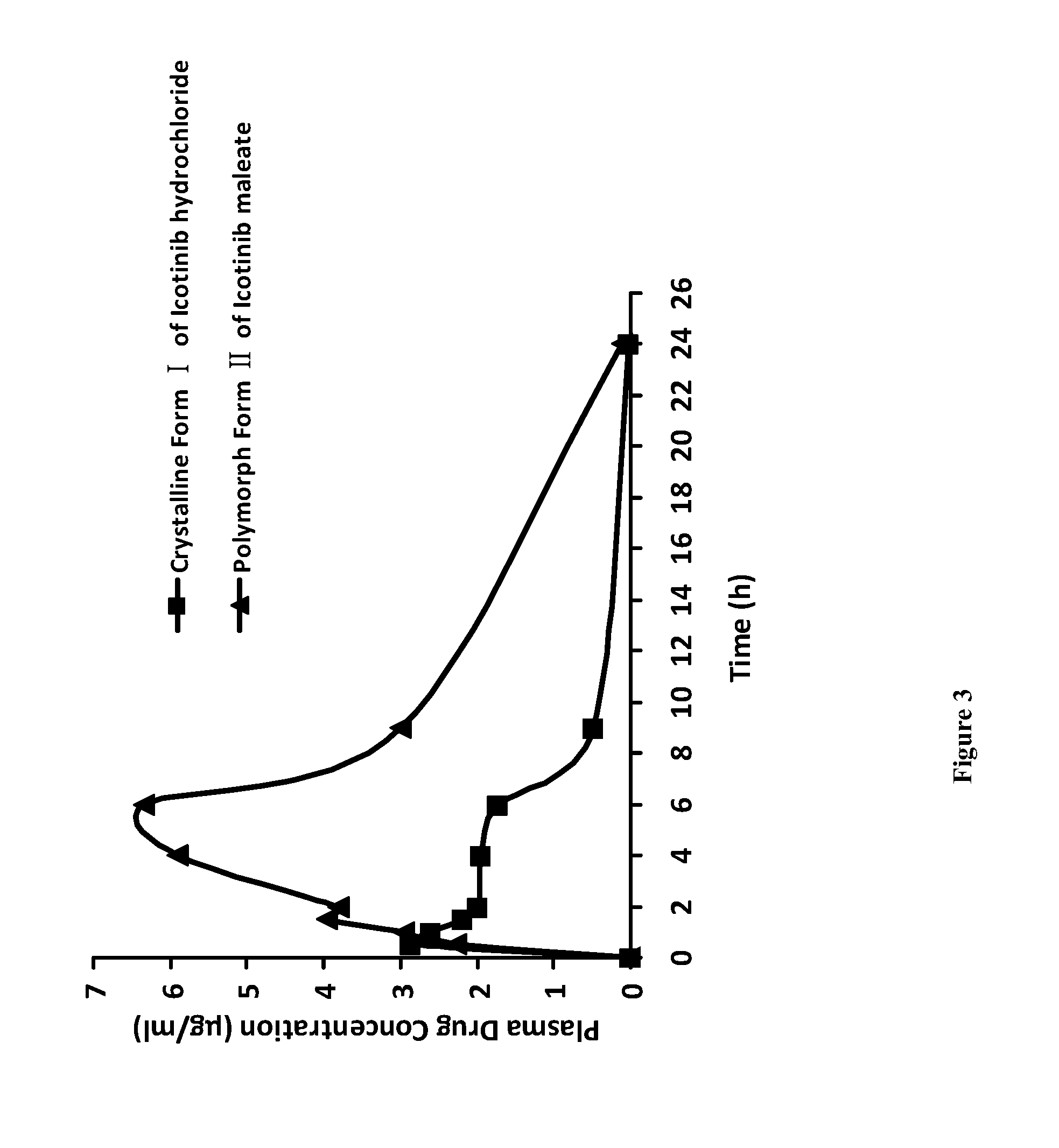
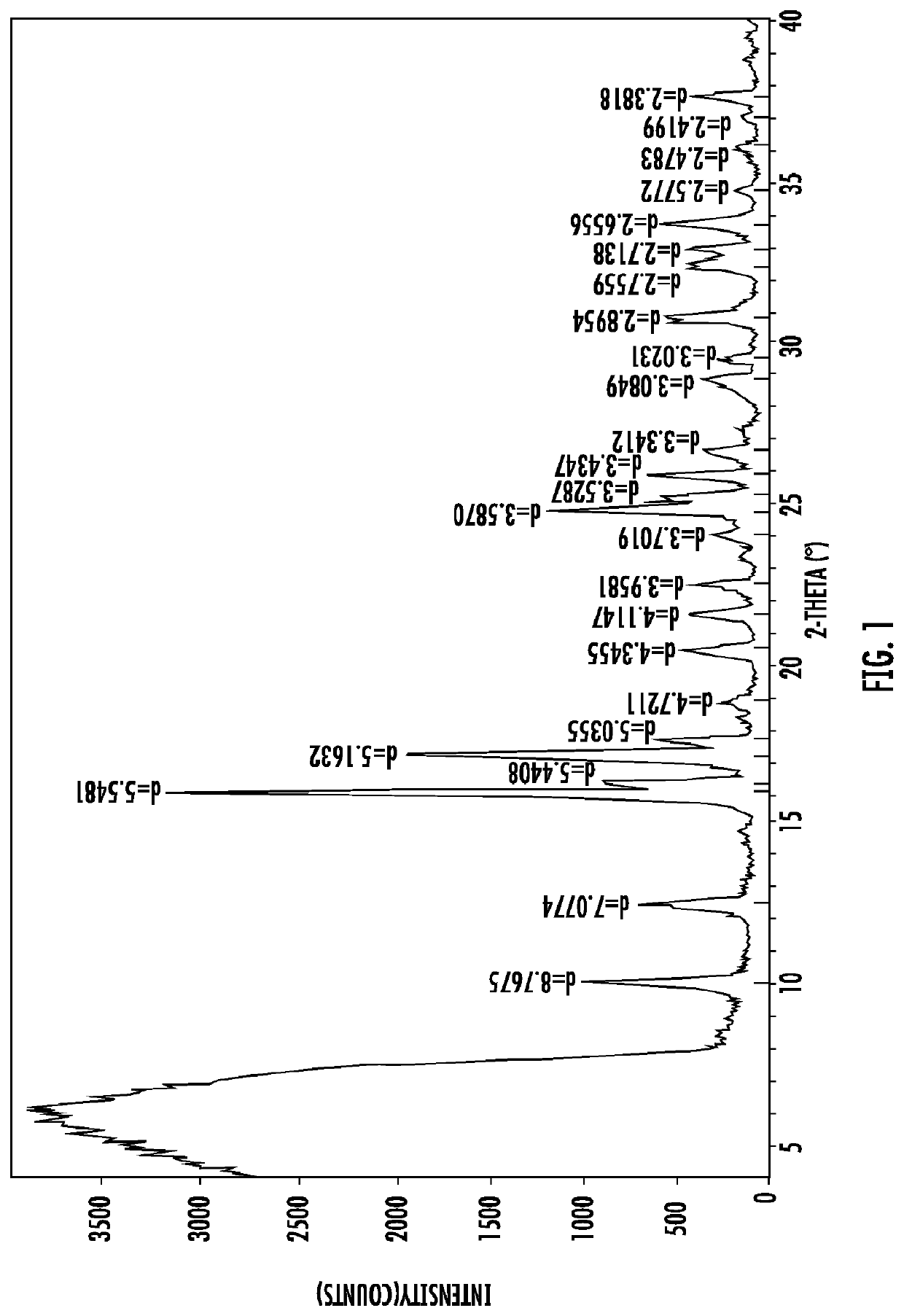
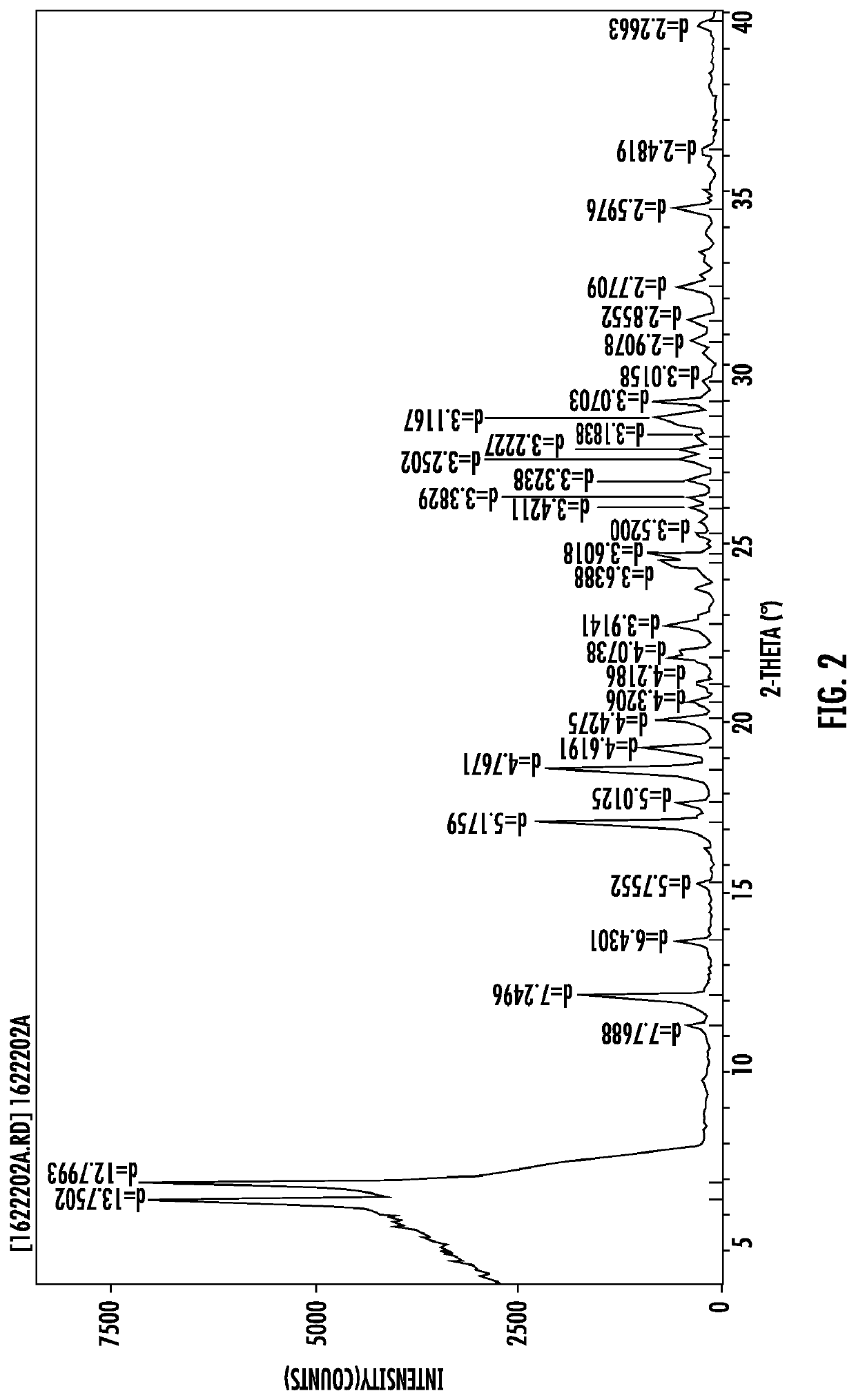
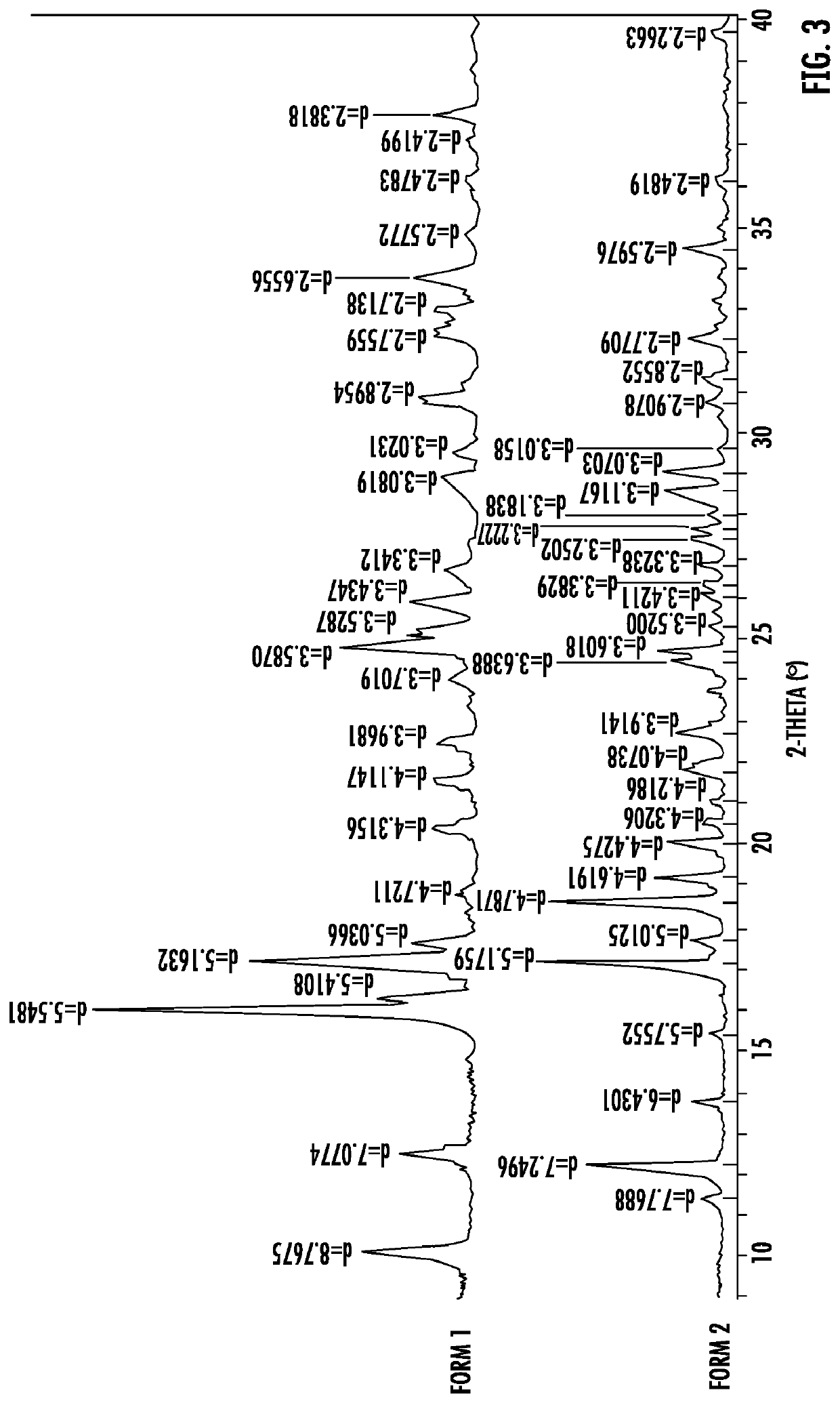
Polymorphic forms of icotinib maleate and uses thereof
Owner:BETTA PHARM CO LTD
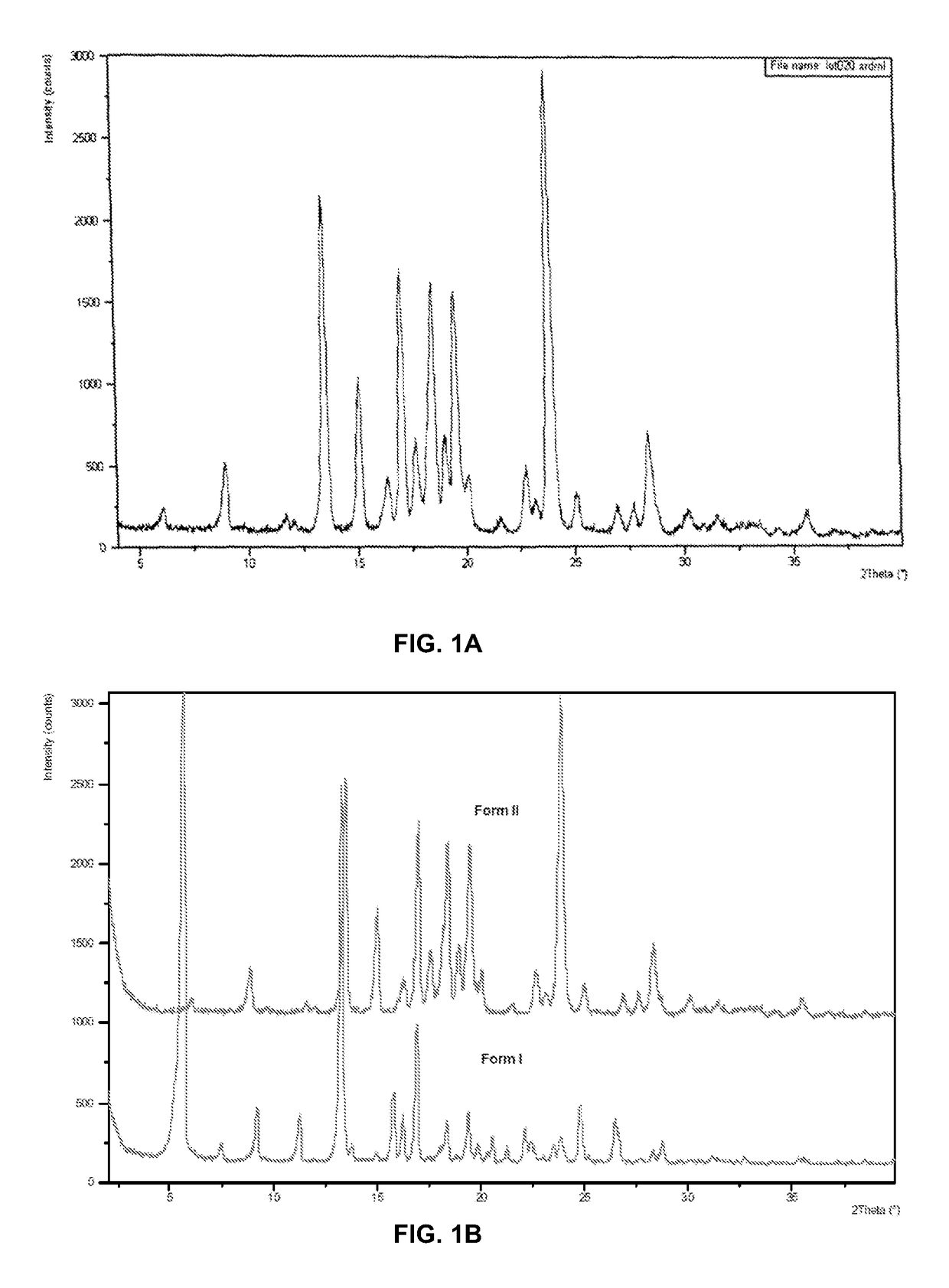
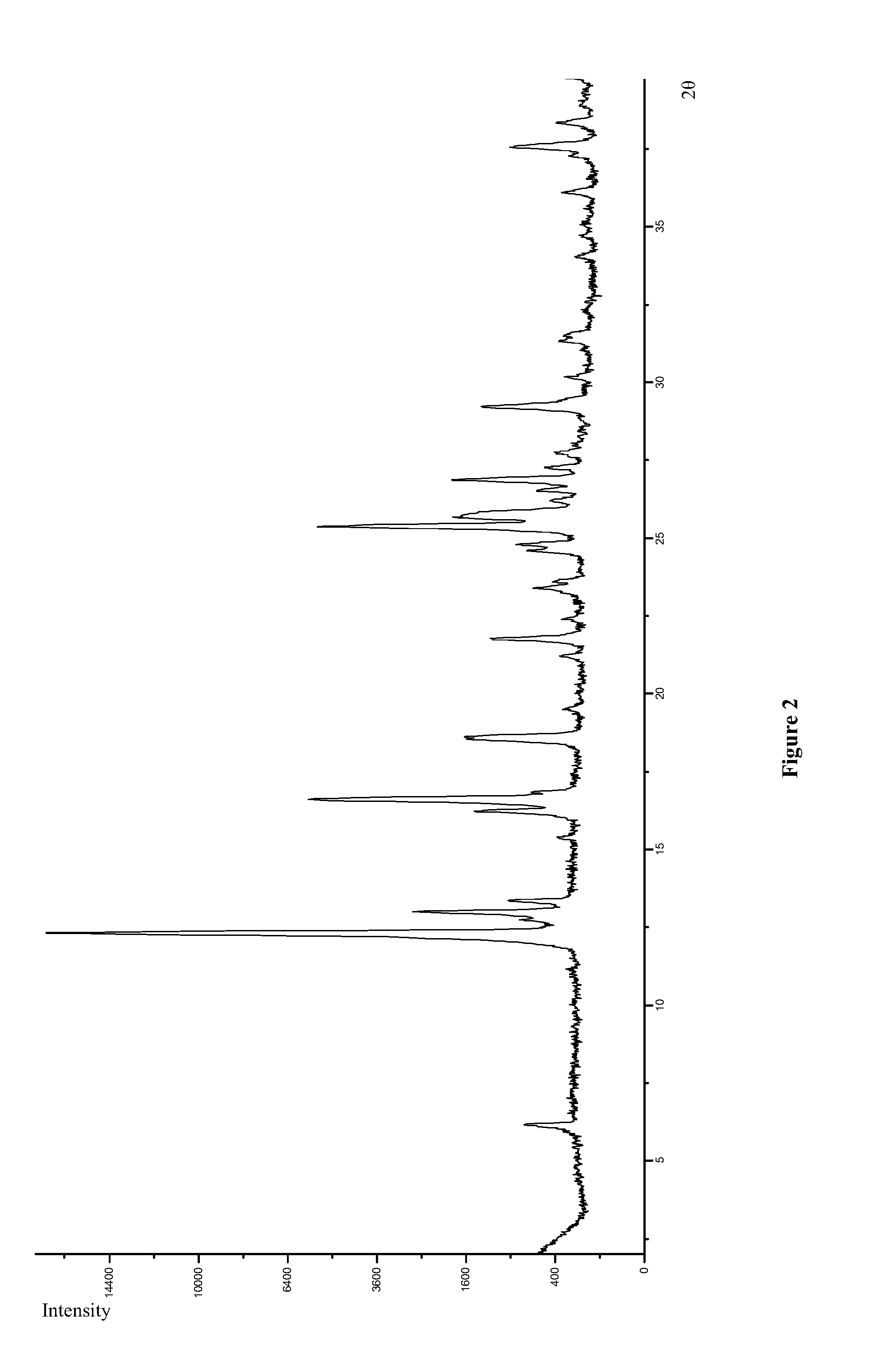
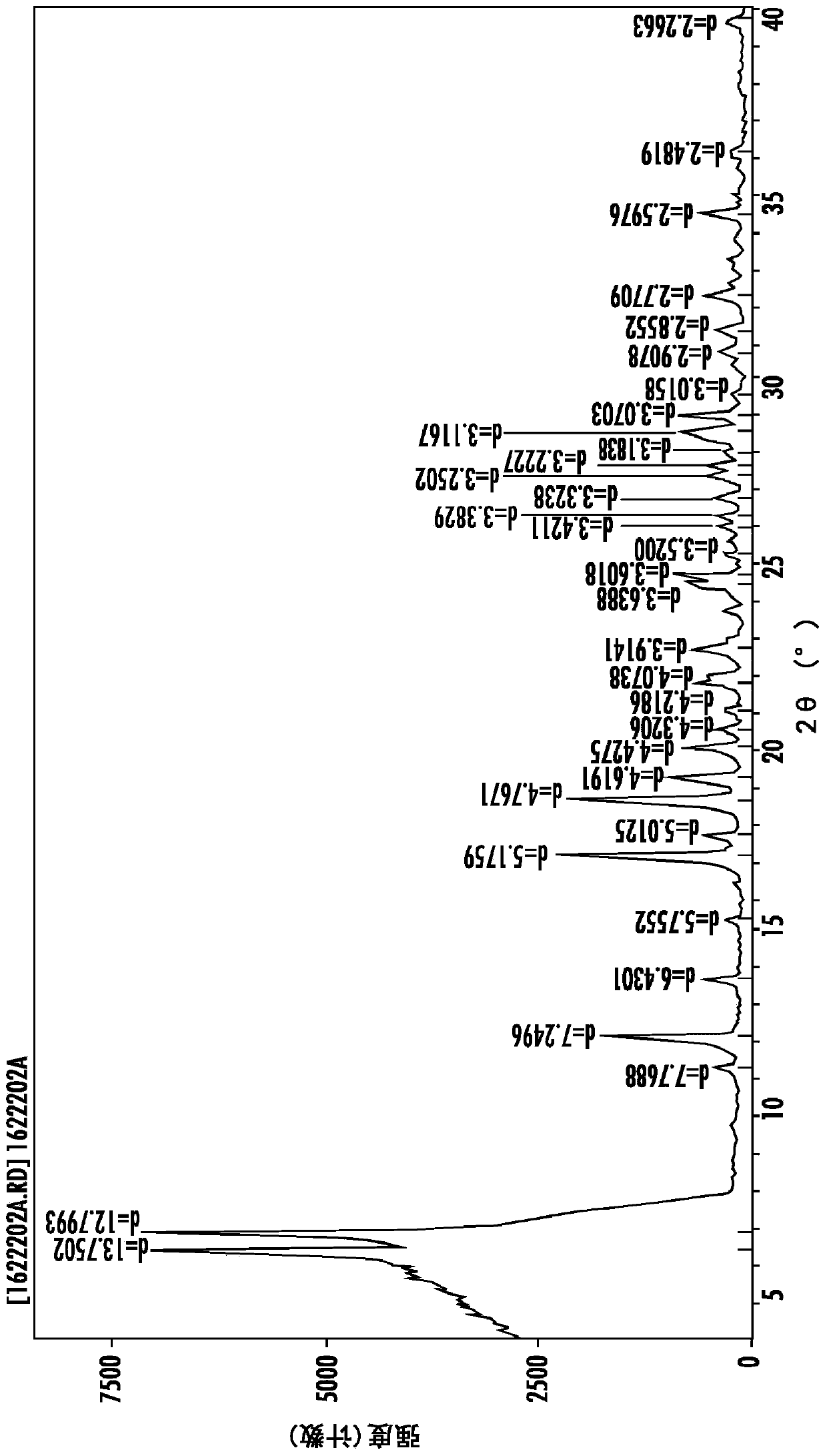
POLYMORPHS OF BENZOATE SALT OF 2-[[6-[(3r)-3-AMINO-1- PIPERIDINYL]-3,4-DIHYDRO-3- METHYL-2,4-DIOXO-1(2H)-PYRIMIDINYL]METHYL]-BENZONITRILE AND METHODS OF USE THEREFORE
Owner:TAKEDA PHARMA CO LTD
Polymorphs of herbicidal sulfonamides
Solid polymorphic forms of sulfentrazone are described. Particularly, a new polymorphic form of sulfentrazone is described herein as sulfentrazone-1, having surprising property advantages over technical sulfentrazone. Processes for the preparation of sulfentrazone-1, herbicidal compositions comprising sulfentrazone-1, and methods of its use are described.
Owner:FMC CORP
Polymorphs of herbicidal sulfonamides
Solid polymorphic forms of sulfentrazone are described. Particularly, a new polymorphic form of sulfentrazone is described herein as sulfentrazone-1, having surprising property advantages over technical sulfentrazone. Processes for the preparation of sulfentrazone-1, herbicidal compositions comprising sulfentrazone-1, and methods of its use are described.
Owner:FMC CORP
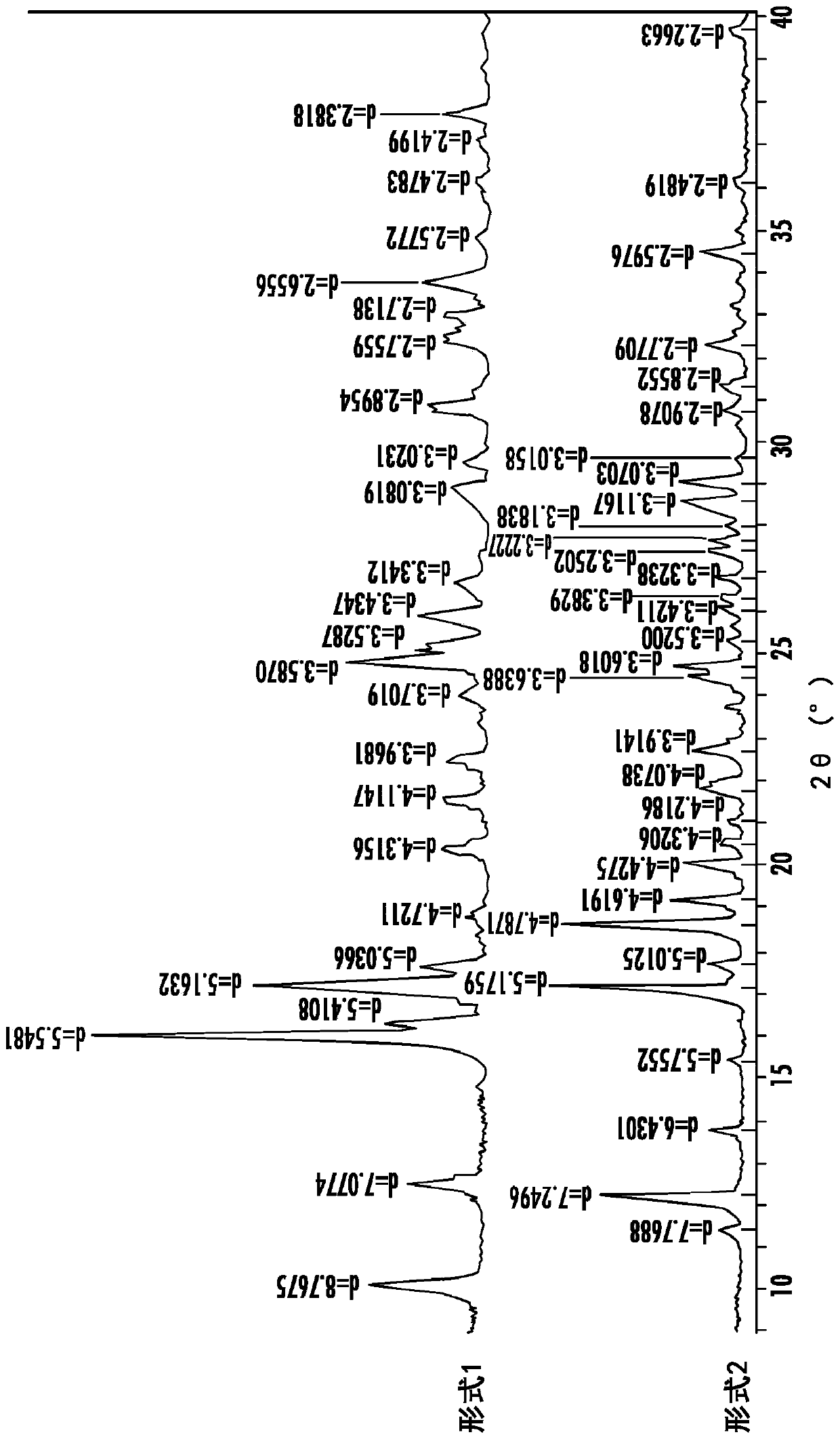
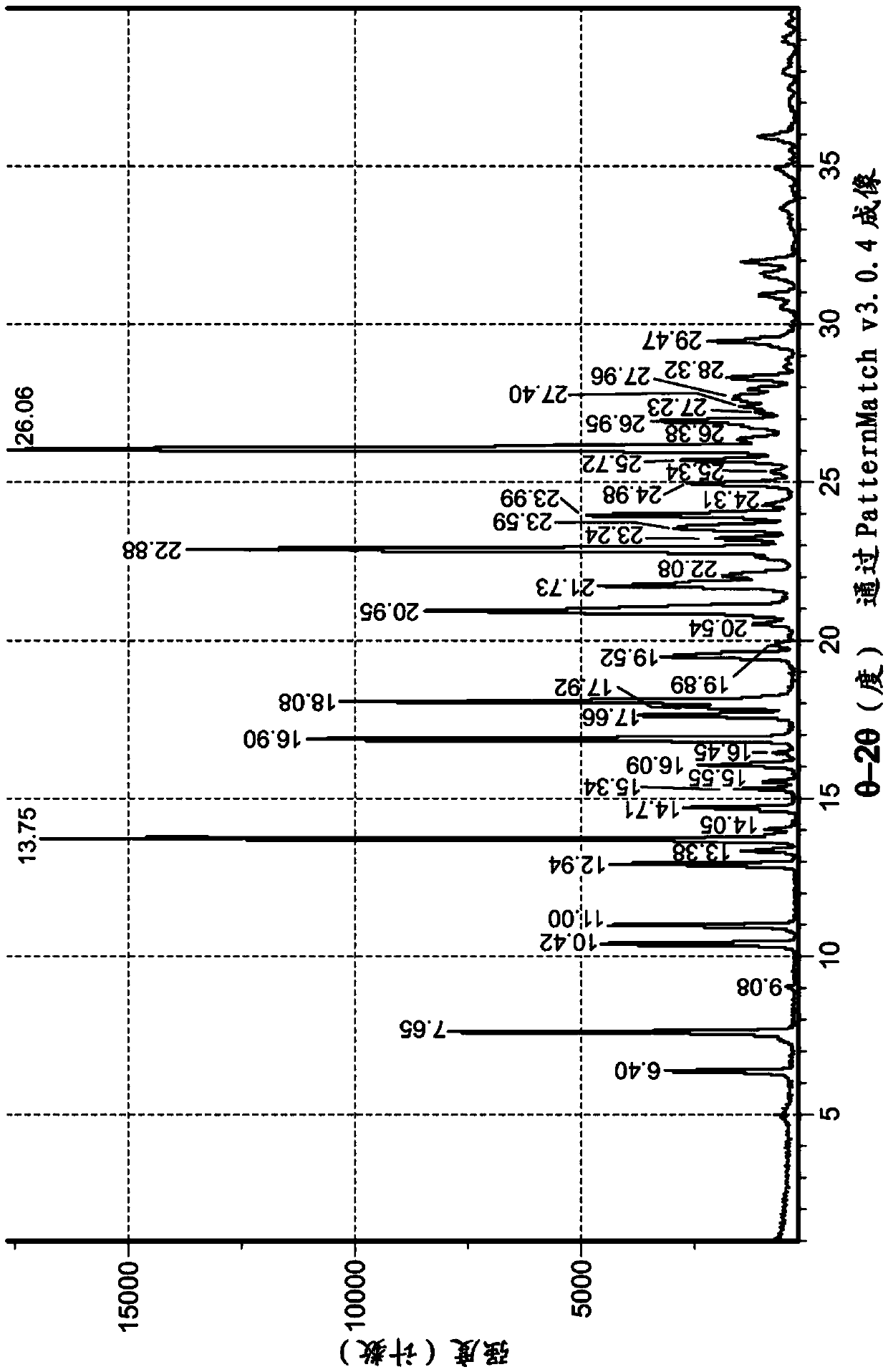
Polymorphs of tartrate salt of 2-[2-(3-(r)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6h-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor
Owner:TAKEDA PHARMA CO LTD
Aprepitant polymorph mixtures
InactiveUS8217039B2Excellent propertyHigh thermodynamic stabilityBiocideOrganic chemistryCrystallizationChemistry
Intimate mixtures of aprepitant crystalline Form I and crystalline Form II, having specific weight ratios of the forms.
Owner:DR REDDYS LAB LTD +1
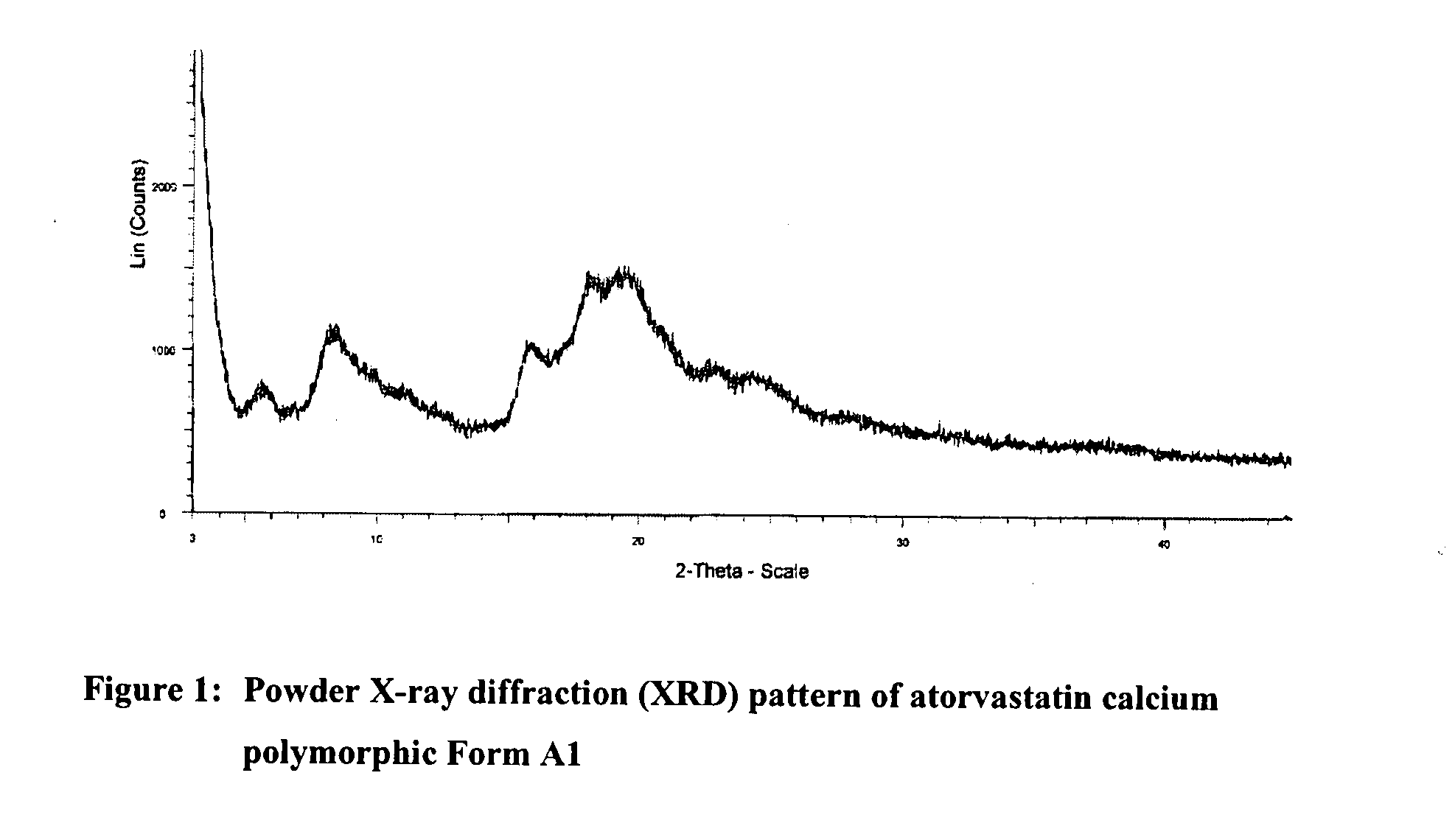
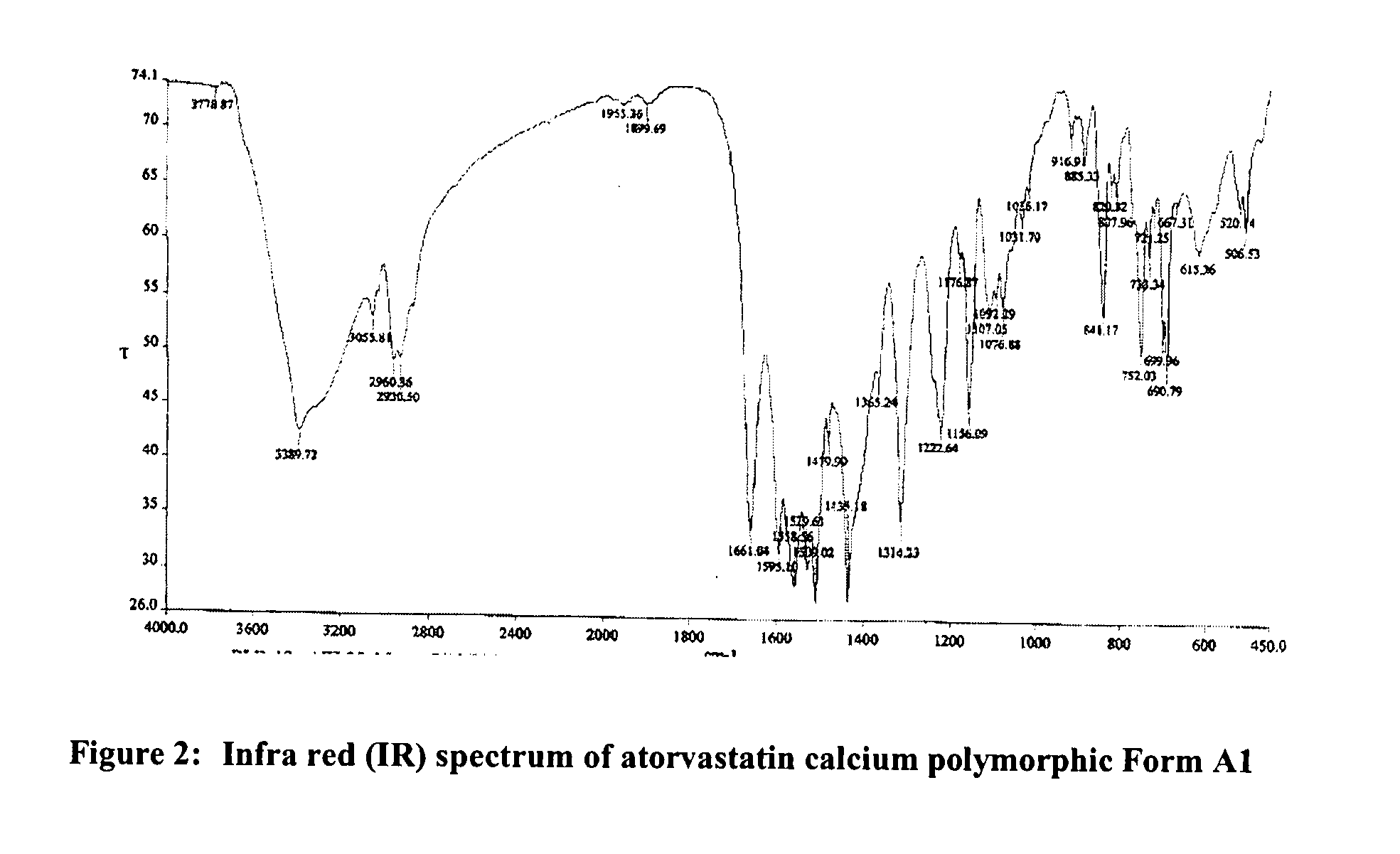
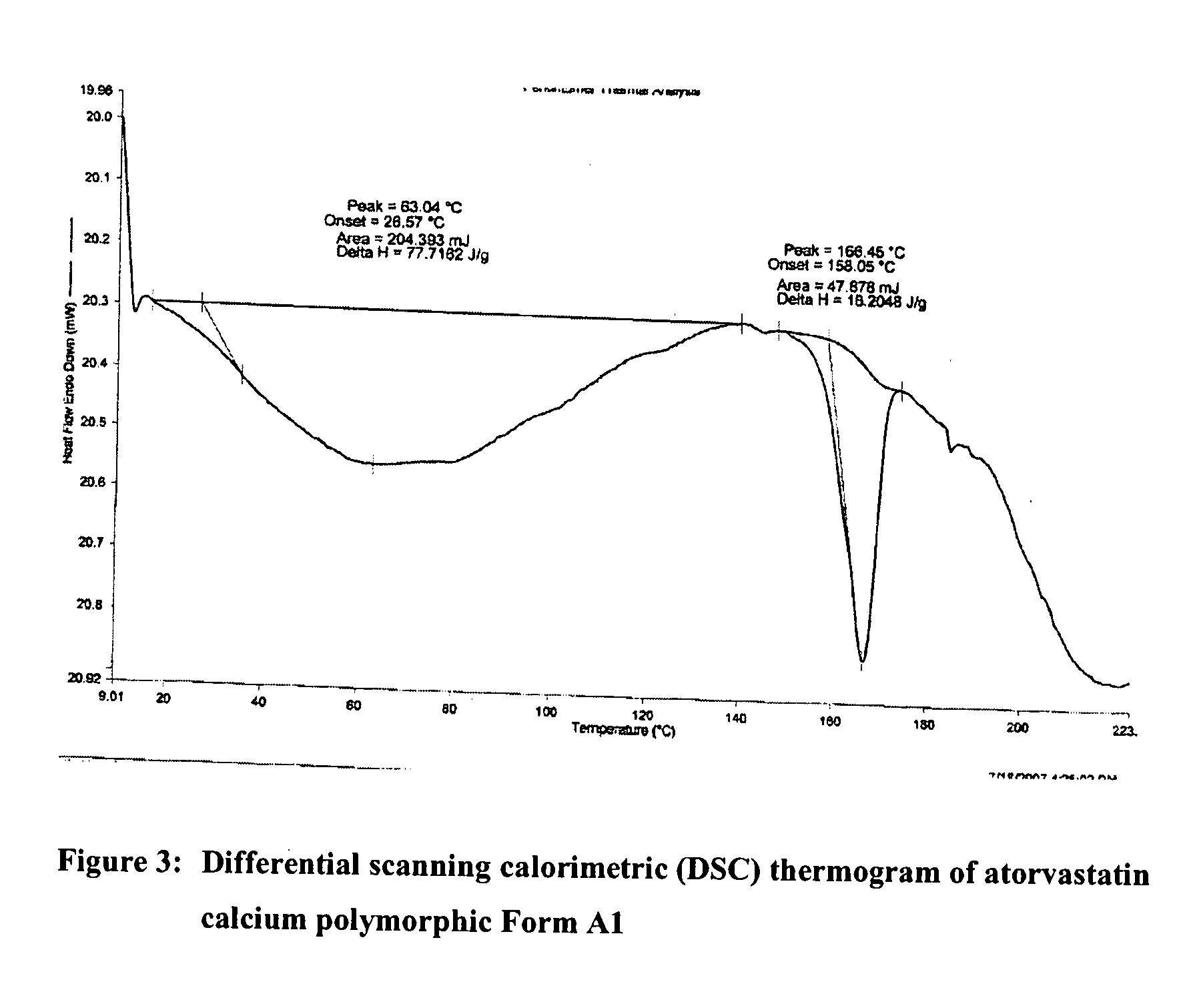
Novel Polymorph of Atorvastatin Calcium and Use Thereof for the Preparation of Amorphous Atorvastatin Calcium
The present invention provides a novel polymorphic form of atorvastatin calcium, designated as form Al, process for preparation, pharmaceutical compositions, and method of treating thereof. The present invention further provides a process for the preparation of highly pure amorphous atorvastatin calcium using the novel atorvastatin calcium form Al. The present invention also relates to novel amorphous form of atorvastatin tert-butyl ester, chemically known as [R-(R*,R*)]-2-(4-fluorophenyl)-[β],[δ]-dihydroxy -5-(1-methylethyl)-3-phenyl-4-(phenylcarbamoyl-1H-pyrrole-1-heptanoicacid tert-butyl ester, process for the preparation, and its application for preparing highly pure atorvastatin and its pharmaceutically acceptable salts thereof. The present invention also relates to use of the novel amorphous atorvastatin tert-butyl ester and novel atorvastatin calcium form al for preparing amorphous atorvastatin calcium.
Owner:ACTAVIS GRP PTC EHF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka.patsnap.com/patent_img/e89c9398-ca99-4dab-9d3b-309edeb3602f/US06903106-20050607-D00001.png)
![Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka.patsnap.com/patent_img/e89c9398-ca99-4dab-9d3b-309edeb3602f/US06903106-20050607-D00002.png)
![Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorph of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka.patsnap.com/patent_img/e89c9398-ca99-4dab-9d3b-309edeb3602f/US06903106-20050607-D00003.png)


![Crystalline form of pyrimidio[6,1-A]isoquinolin-4-one compound Crystalline form of pyrimidio[6,1-A]isoquinolin-4-one compound](https://images-eureka.patsnap.com/patent_img/e12b654e-9fa0-4475-aa95-1ee883682144/DEST_PATH_HDA00003503009600011.PNG)
![Crystalline form of pyrimidio[6,1-A]isoquinolin-4-one compound Crystalline form of pyrimidio[6,1-A]isoquinolin-4-one compound](https://images-eureka.patsnap.com/patent_img/e12b654e-9fa0-4475-aa95-1ee883682144/DEST_PATH_HDA00003503009600012.PNG)
![Crystalline form of pyrimidio[6,1-A]isoquinolin-4-one compound Crystalline form of pyrimidio[6,1-A]isoquinolin-4-one compound](https://images-eureka.patsnap.com/patent_img/e12b654e-9fa0-4475-aa95-1ee883682144/DEST_PATH_HDA00003503009600021.PNG)
![POLYMORPHS OF BENZOATE SALT OF 2-[[6-[(3r)-3-AMINO-1- PIPERIDINYL]-3,4-DIHYDRO-3- METHYL-2,4-DIOXO-1(2H)-PYRIMIDINYL]METHYL]-BENZONITRILE AND METHODS OF USE THEREFORE POLYMORPHS OF BENZOATE SALT OF 2-[[6-[(3r)-3-AMINO-1- PIPERIDINYL]-3,4-DIHYDRO-3- METHYL-2,4-DIOXO-1(2H)-PYRIMIDINYL]METHYL]-BENZONITRILE AND METHODS OF USE THEREFORE](https://images-eureka.patsnap.com/patent_img/18846759-3a6f-41cb-b004-99f44b0f5645/US20090306379A1-20091210-D00001.png)
![POLYMORPHS OF BENZOATE SALT OF 2-[[6-[(3r)-3-AMINO-1- PIPERIDINYL]-3,4-DIHYDRO-3- METHYL-2,4-DIOXO-1(2H)-PYRIMIDINYL]METHYL]-BENZONITRILE AND METHODS OF USE THEREFORE POLYMORPHS OF BENZOATE SALT OF 2-[[6-[(3r)-3-AMINO-1- PIPERIDINYL]-3,4-DIHYDRO-3- METHYL-2,4-DIOXO-1(2H)-PYRIMIDINYL]METHYL]-BENZONITRILE AND METHODS OF USE THEREFORE](https://images-eureka.patsnap.com/patent_img/18846759-3a6f-41cb-b004-99f44b0f5645/US20090306379A1-20091210-D00002.png)
![POLYMORPHS OF BENZOATE SALT OF 2-[[6-[(3r)-3-AMINO-1- PIPERIDINYL]-3,4-DIHYDRO-3- METHYL-2,4-DIOXO-1(2H)-PYRIMIDINYL]METHYL]-BENZONITRILE AND METHODS OF USE THEREFORE POLYMORPHS OF BENZOATE SALT OF 2-[[6-[(3r)-3-AMINO-1- PIPERIDINYL]-3,4-DIHYDRO-3- METHYL-2,4-DIOXO-1(2H)-PYRIMIDINYL]METHYL]-BENZONITRILE AND METHODS OF USE THEREFORE](https://images-eureka.patsnap.com/patent_img/18846759-3a6f-41cb-b004-99f44b0f5645/US20090306379A1-20091210-D00003.png)
![Polymorphs of tartrate salt of 2-[2-(3-(r)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6h-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor Polymorphs of tartrate salt of 2-[2-(3-(r)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6h-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor](https://images-eureka.patsnap.com/patent_img/876b6890-126e-4dda-bdbd-ee014430707e/US20070066636A1-20070322-D00001.png)
![Polymorphs of tartrate salt of 2-[2-(3-(r)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6h-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor Polymorphs of tartrate salt of 2-[2-(3-(r)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6h-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor](https://images-eureka.patsnap.com/patent_img/876b6890-126e-4dda-bdbd-ee014430707e/US20070066636A1-20070322-D00002.png)
![Polymorphs of tartrate salt of 2-[2-(3-(r)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6h-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor Polymorphs of tartrate salt of 2-[2-(3-(r)-amino-piperidin-1-yl)-5-fluoro-6-oxo-6h-pyrimidin-1-ylmethyl]-benzonitrile and methods of use therefor](https://images-eureka.patsnap.com/patent_img/876b6890-126e-4dda-bdbd-ee014430707e/US20070066636A1-20070322-D00003.png)