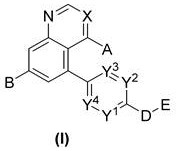
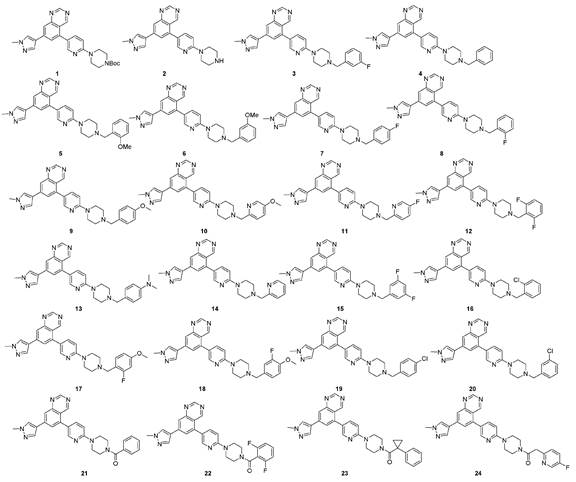
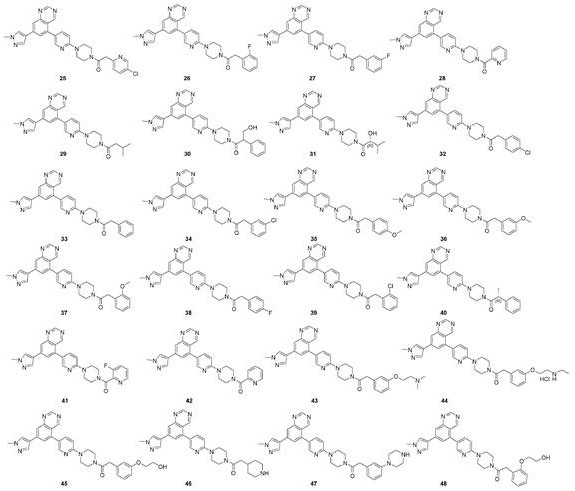
New compound used as rearranged during transfection kinase inhibitor
A technology of compounds and derivatives, applied in the treatment or prevention of related diseases mediated by RET kinase, rearrangement during transfection (RET) kinase inhibitor, in the field of preparing the compounds described below, can solve the problem of poor RET selectivity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0511] 4-(5-(7-(1-methyl-1 H -pyrazol-4-yl)quinazolin-5-yl)pyridin-2-yl)piperazine-1-carboxylic acid tert-butyl ester
[0512]
[0513]
[0514] first step
[0515] 4-Bromo-2-chloro-6-fluorobenzene(formaldehyde)
[0516] Add anhydrous tetrahydrofuran (400 mL) into a 500 mL three-necked flask, add lithium diisopropylamide (0.26mol, 130 mL, 2 M in THF) at -78 °C, and slowly add compound 1-bromo-3-chloro - A solution of 5-fluorobenzene 1a (25.00 g, 0.12 mol) in anhydrous THF (100 mL). Stir at -78°C for 1 hour, add dropwise N , N -Dimethylformamide (17.50 g, 0.24 mol), -78°C Stirring was continued for 2 hours. Quenched with saturated ammonium chloride solution at low temperature, desolvated under reduced pressure to remove part of the solvent, extracted with dichloromethane (200 mL × 3), washed the organic phase with saturated brine (500 mL), and washed with water (500 mL). The organic phase was dried with anhydrous sodium sulfate, filtered to remove the desiccant, and...
Embodiment 2
[0532] 7-(1-Methyl-1 H -Pyrazol-4-yl)-5-(6-(piperazin-1-yl)pyridin-3-yl)quinazoline
[0533]
[0534]
[0535] Compound 4-(5-(7-(1-methyl-1 H -pyrazol-4-yl)quinazolin-5-yl)pyridin-2-yl)piperazine-1-carboxylate tert-butyl ester 1 (0.50 g, 1.06 mmol) was dissolved in methanol (5 mL), and hydrochloric acid was added Methanol (80 mmol, 4 M, 20 mL), stirred at room temperature for 3 hours, desolvated under reduced pressure to obtain the crude product, the residue was dissolved in water (20 mL), added saturated aqueous sodium bicarbonate to pH = 8-9, distilled with di Chloromethane (20 mL × 5) was extracted, the organic phase was dried with anhydrous sodium sulfate, the desiccant was removed by filtration, and the target compound 7-(1-methyl-1 H -pyrazol-4-yl)-5-(6-(piperazin-1-yl)pyridin-3-yl)quinazoline 2 (0.37 g, yellow solid), yield: 94%.
[0536] MS m / z(ESI): 372 [M+1];
[0537] 1 H NMR (400 MHz, CDCl 3 ) δ 9.37 (s, 1H), 9.28 (s, 1H), 8.36 (s, 1H), 8.06(s, 1H), 7.96...
Embodiment 3
[0539] 5-(6-(4-(3-fluorobenzyl)piperazin-1-yl)pyridin-3-yl)-7-(1-methyl-1 H -pyrazol-4-yl)quinazoline
[0540]
[0541]
[0542] Compound 7-(1-methyl-1 H -pyrazol-4-yl)-5-(6-(piperazin-1-yl)pyridin-3-yl)quinazoline 2 (10 mg, 0.02 mmol), m-fluorobenzaldehyde (5 mg, 0.04 mmol ) and methanol (1 mL), and stirred at room temperature for 5 minutes. Then sodium triacetoxyborohydride (11 mg, 0.04 mmol) was added and stirred at room temperature for 12 hours. The reaction solution was quenched with saturated ammonium chloride (2 mL), extracted with ethyl acetate (5 mL), the organic phase was dried over anhydrous sodium sulfate, the desiccant was removed by filtration, and the crude product was precipitated under reduced pressure, and the residue was prepared with silica gel Plate purification (dichloromethane: methanol = 12: 1 ) to obtain the target product 5-(6-(4-(3-fluorobenzyl)piperazin-1-yl)pyridin-3-yl)-7-(1 -Methyl-1 H -pyrazol-4-yl)quinazoline 3 (7 mg, yellow solid), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com