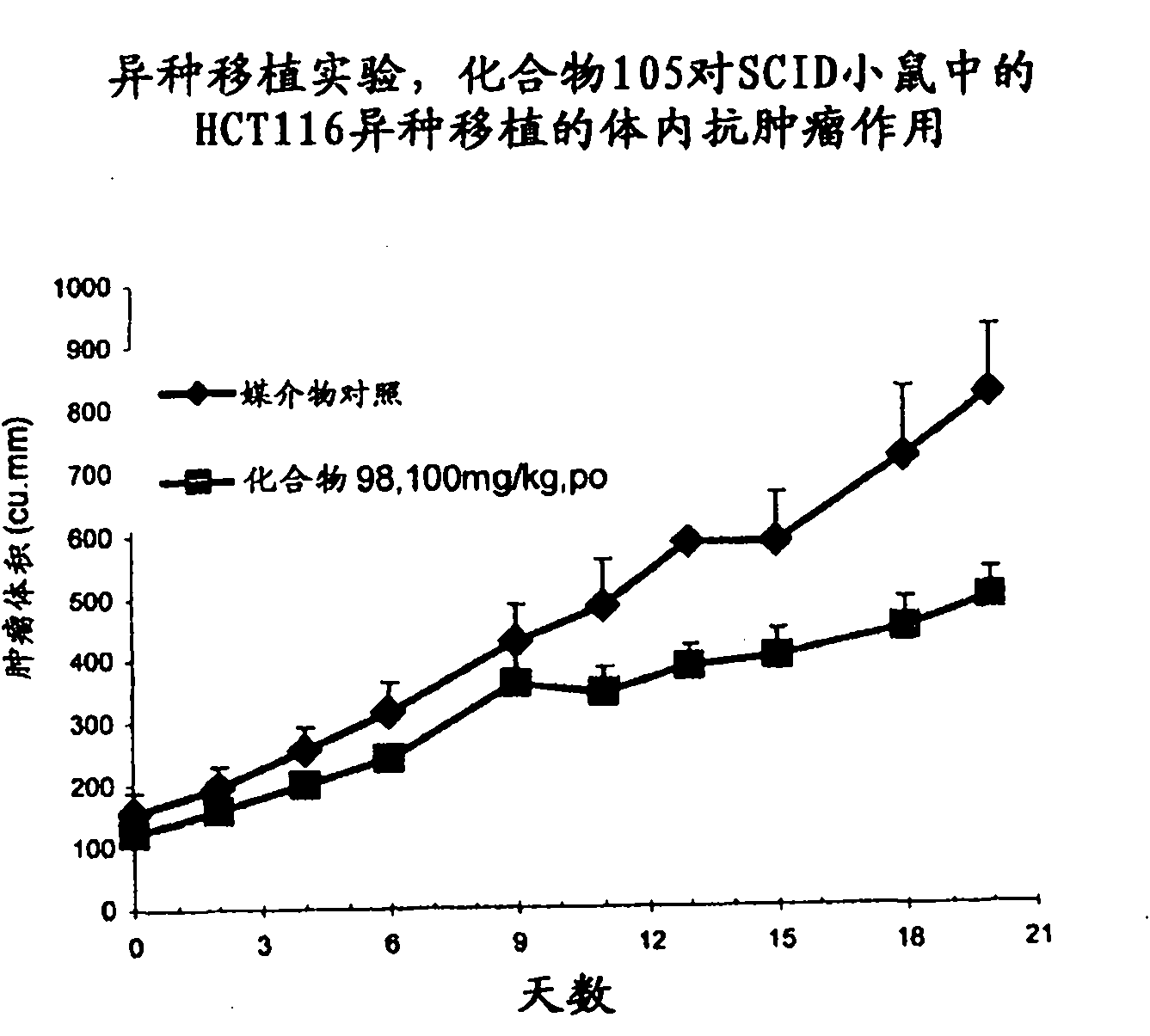
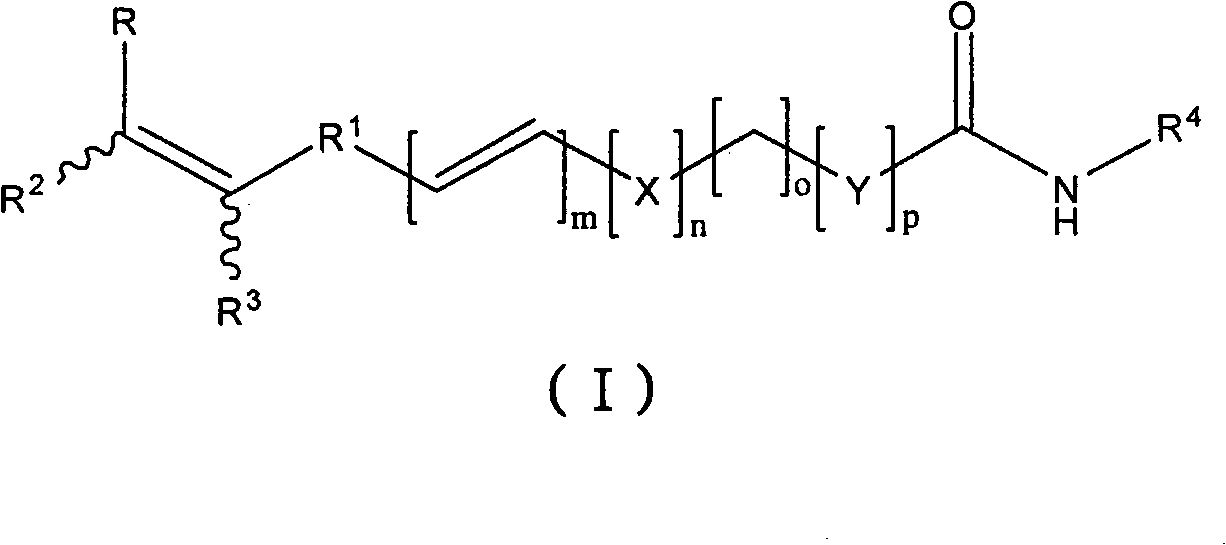
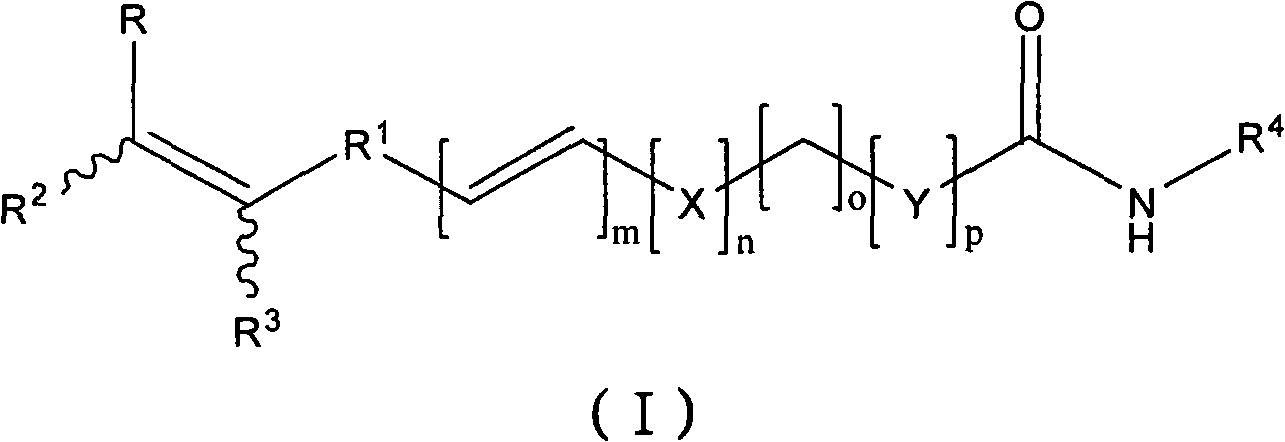
Histone deacetylase inhibitors
A group and aryl technology, applied in the field of histone deacetylase inhibitors, can solve the problem that DNA is difficult to regulate transcription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0292] N-cyclopropyl-2-(4-fluorophenyl)-3-(4-((E)-3-(hydroxyamino)-3-oxoprop-1-en-1-yl)phenyl) Synthesis of Acrylamide.
[0293]
[0294] Step-I
[0295] Preparation of (E)-3-(4-formylphenyl)methyl acrylate
[0296]
[0297] A suspension of (E)-3-(4-formylphenyl)acrylic acid (2 g, 10.5 mmol) in methanol (30 mL) was cooled to 5 °C, then concentrated H was added with stirring. 2 SO 4 (3 mL) and heated at 60 °C for 2 hours. The solvent was removed by evaporation and the obtained compound was stirred with water (100 mL) for 15 minutes. The precipitated white solid was filtered, washed with water (300 mL) and dried to obtain pure product (1.9 g, 86% yield).
[0298] Step-II
[0299] Preparation of 2-(4-fluorophenyl)-3-(4-((E)-3-methoxy-3-oxoprop-1-en-1-yl)phenyl)acrylic acid
[0300]
[0301] Under stirring, a mixture of 4-fluorophenylacetic acid (2.5g, 13.2mmol) and methyl (E)-3-(4-formylphenyl)acrylate (2.03g, 13.2mmol) was mixed with acetic anhydride (8mL ) ...
Embodiment 55
[0330] Synthesis of (1E)-3-(4-(3-(cyclopropylamino)-2-(4-fluorophenyl)prop-1-en-1-yl)phenyl)-N-hydroxyacrylamide
[0331]
[0332] Step-I
[0333] Preparation of methyl 3-(1E)-(4-(2-(4-fluorophenyl)-3-hydroxyprop-1-en-1-yl)phenyl)acrylate
[0334]
[0335] Under constant stirring at 5°C, to 2-(4-fluorophenyl)-3-(4-(3-(E)-methoxy-3-oxoprop-1-en-1-yl)benzene To a suspension of acrylic acid (2 g, 6.1 mmol, prepared according to the procedure described in Example 1 step-II) in THF (10 mL), triethylamine (0.85 mL, 6.67 mmol) was added. To this solution was added dropwise methyl chloroformate (0.53 mL, 6.67 mmol) at 5°C over a period of 30 minutes, and stirred at the same temperature for 30 minutes. To the reaction mixture, sodium borohydride (0.9 g, 24.5 mmol) was added in one portion and methanol (5 mL) was added dropwise with stirring, and the reaction mixture was stirred at 30° C. for 2 hours. After the reaction was finished, the reaction mixture was diluted with ethy...
Embodiment 59
[0352] N-cyclopropyl-3-(4-((1E)-3-(2-aminophenylamino)-3-oxoprop-1-en-1-ylphenyl)-2-(4-fluoro Synthesis of phenyl)-acrylamide
[0353]
[0354] Step-I
[0355] Preparation of 3-(1E)-(4-(3-cyclopropylamino)-2-(4-fluorophenyl)-3-oxoprop-1-en-1-yl)phenyl)acrylic acid
[0356]
[0357] To 3-(1E)-(4-(3-(cyclopropylamino)-2-(4-fluorophenyl)-3-oxoprop-1-en-1-yl)phenyl)methyl acrylate (1 g, 2.7 mmol) in methanol (20 mL) was added a solution of NaOH (0.44 g, 4.4 mmol) in water (1 mL). The reaction mixture was stirred at 70°C for 2 hours. Subsequently, the solvent was completely removed by evaporation, diluted with water (50 mL) and extracted with ethyl acetate (2x50 mL). The aqueous layer was acidified to pH 2 with diluted aqueous HCl (1 : 1 ) and allowed to stand at 4°C for 30 minutes, the precipitated solid was filtered and dried in vacuo to give the expected product as a white solid (0.67 g, 70 %Yield).
[0358] Step-II
[0359] N-cyclopropyl-3-(4-((1E)-3-(2-aminoph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com