Polymorphs of herbicidal sulfonamides
A polymorphic, sulfentrazone technology, applied in the field of polymorphic forms of herbicidal sulfonamide, can solve problems such as solvent exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
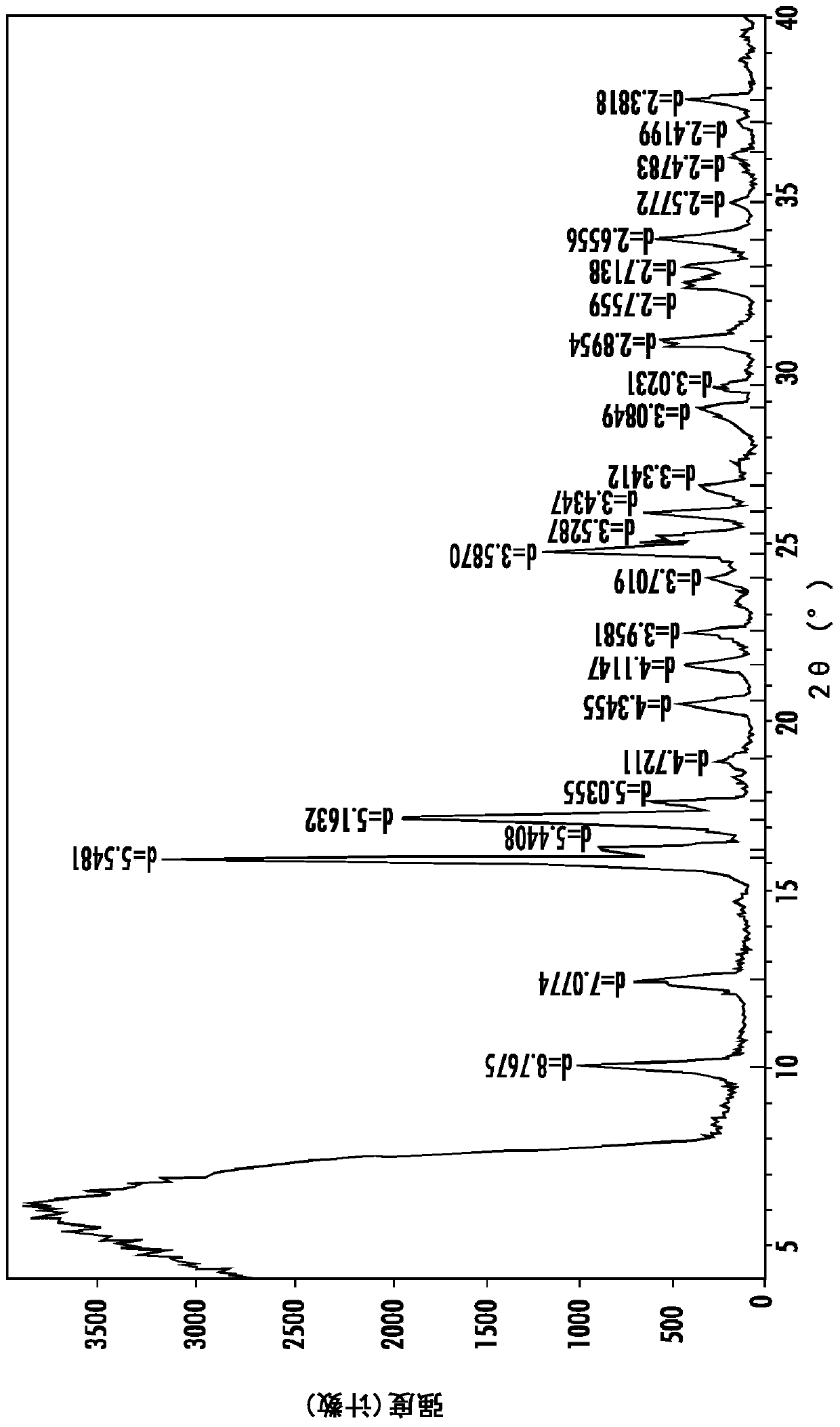
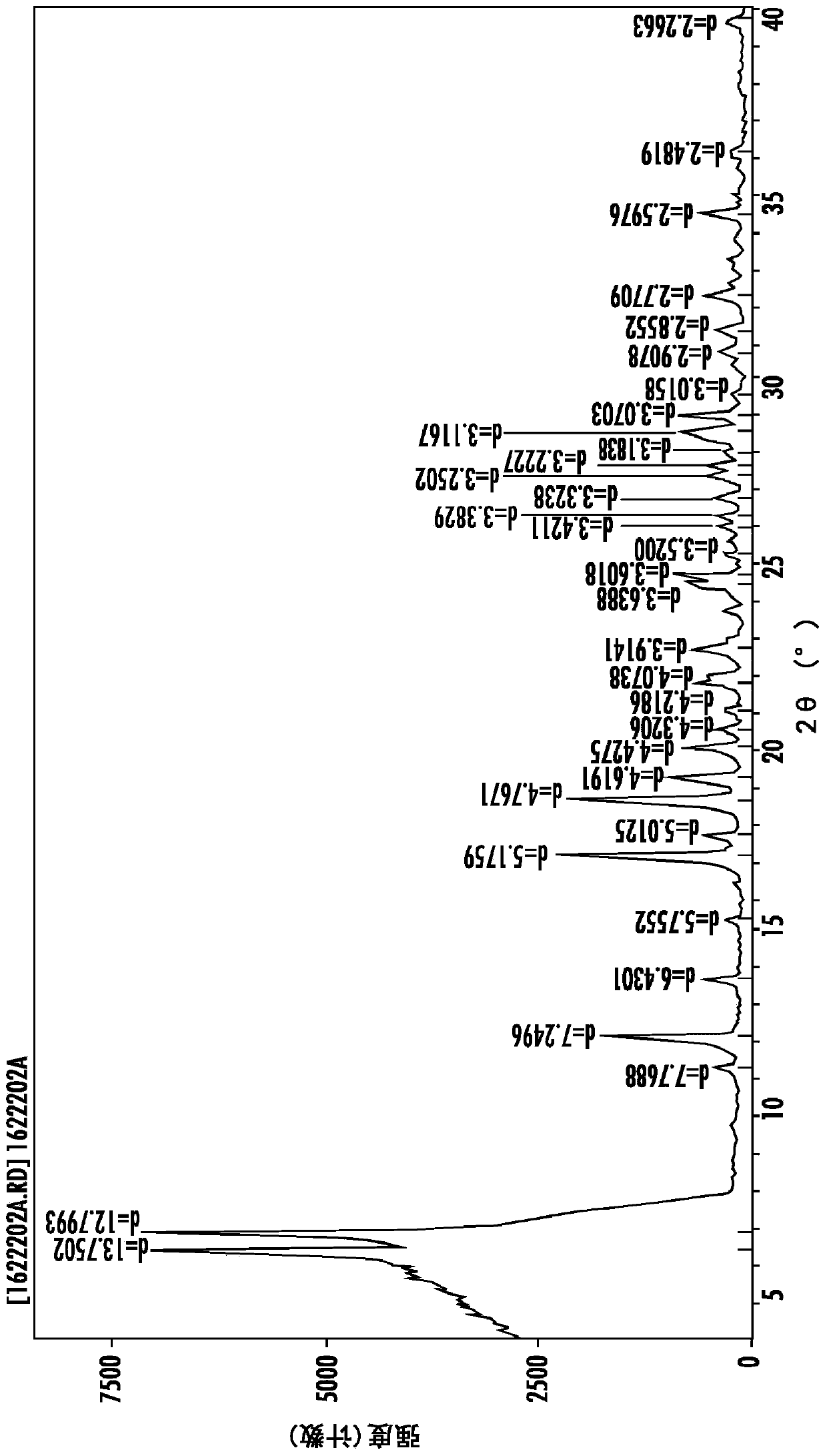
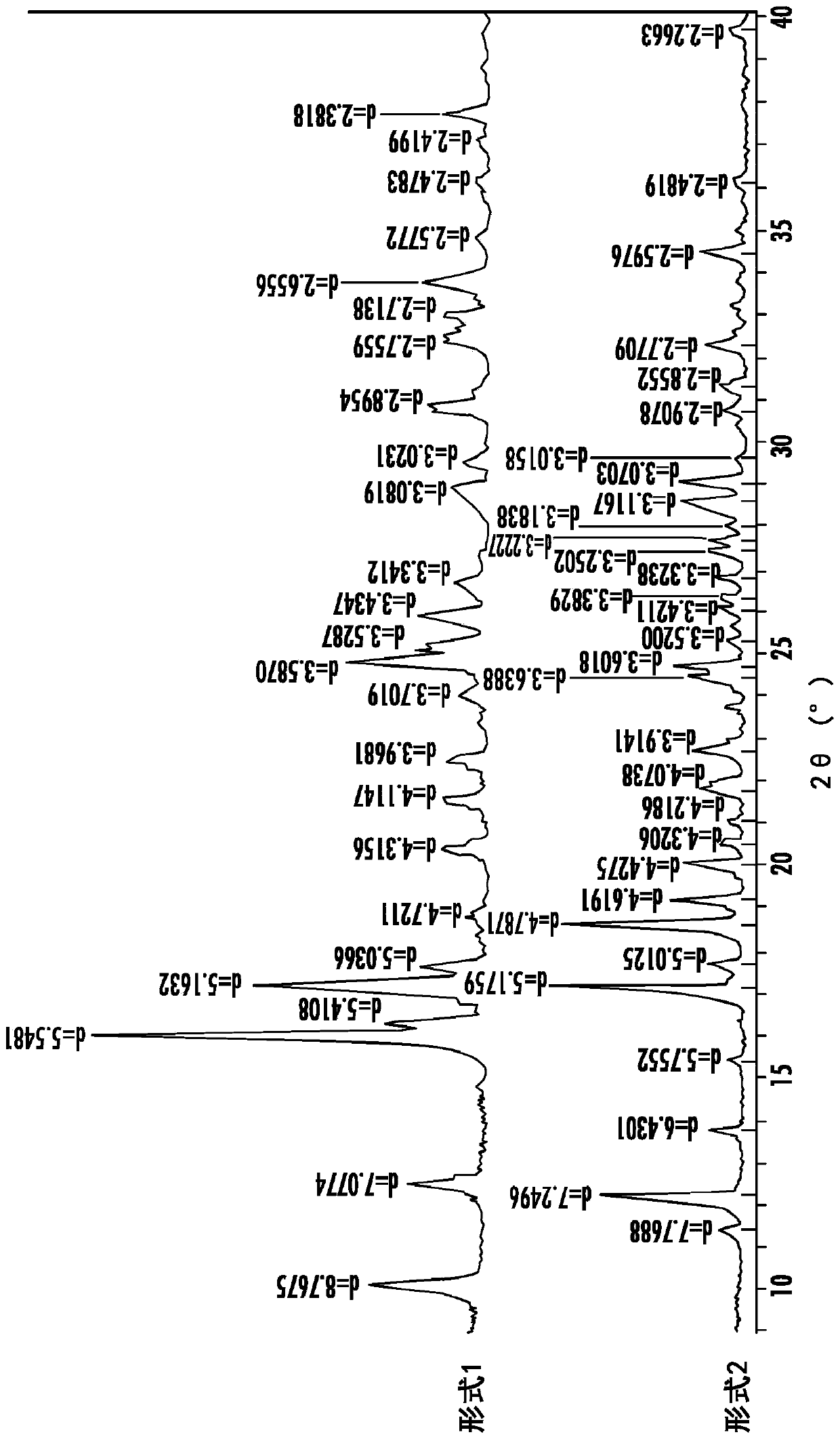
Image
Examples
Embodiment
[0145] Preparation of polymorphs
[0146] Sulfentrazone (Formula (I)) is generally prepared as described in US Patent 7,169,952.
[0147]The following procedure is taken from U.S. Patent 7,169,952 and is a typical procedure used herein for the preparation of technical grade sulfentrazone (Form 2), where possible variations are related to processing conditions such as batch size, time, temperature, etc.:
[0148] This example illustrates the preparation of N-[2,4-dichloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo- Scheme for 1H-1,2,4-triazol-1-yl]phenyl]methanesulfonamide.
[0149] Under a nitrogen atmosphere, 262 lbs of 16 wt% 1-(5-amino-2,4-dichlorophenyl)-4,5-dihydro-4-difluoromethane was charged to a 50-gallon glass-lined reactor A solution of 3-methyl-1,2,4-triazol-5(1H)-one in toluene. The solution was stirred and heated to about 105-115°C. During heating, the nitrogen atmosphere was stopped and the reaction vessel was sealed under a vacuum of about 750-780 mmH...
preparation Embodiment
[0165] The sulfentrazone-1 obtained as above was formulated into a suspension concentrate and a wettable powder.
[0166] 3a. Preparation of formulations of sulfentrazone-1 in the form of suspension concentrates
Embodiment 1
[0168]
[0169]
[0170] Mix water with Tergitol TM XD and Tergitol TM XH mix until they are completely dissolved. Join Dextrol TM OC-180. The mixture was transferred to an attritor and sulfentrazone-1 was added. The slurry was milled using 0.5 mm stainless steel beads until the particle size was reduced to 13 μd90. The milled slurry is separated from the grinding media and the slurried in propylene glycol M and GXL is added to the mix.
[0171] 3b. Formulation of sulfentrazone-1 in wettable powder form
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com