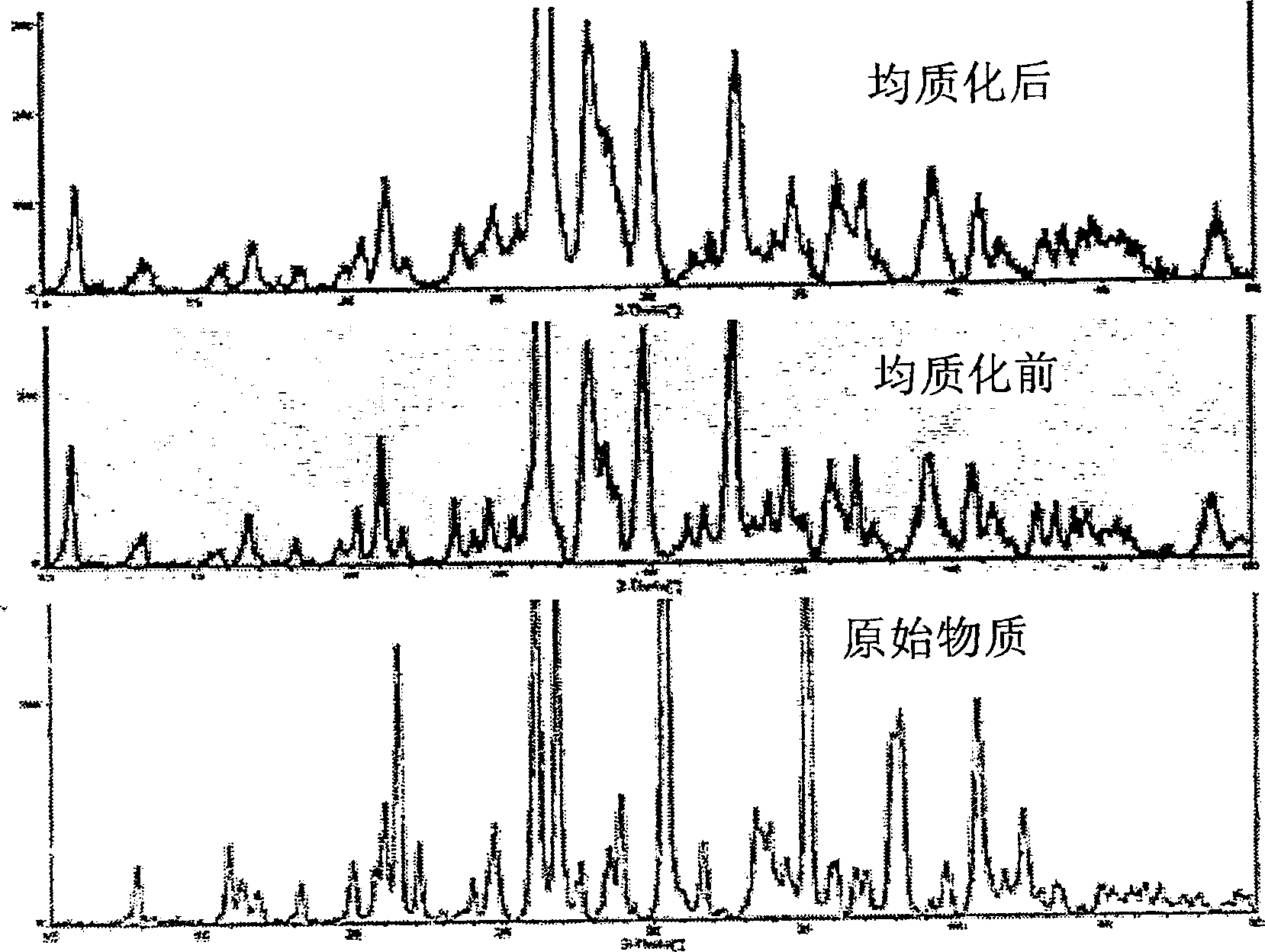
Preparation of submicron sized particles with polymorph control and new polymorph of itraconazole
A polymorphic, particle technology, applied to water-insoluble drugs, 6,596 discloses an organic phase in which a pharmacological activator is dispersed and a nanoparticle field of 20 nanometers, which can solve problems such as drug overtime growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Preparation of itraconazole suspension by homogenization
[0109] Add 1680mL of water to a 3-liter flask, heat the liquid to 60°C-65°C, slowly add 44g of Pluronic F-68 (poloxamer 188), and 12g of sodium deoxycholate, each time Stir after adding the solids. After complete addition of solids, stir at 60°C-65°C for 15 minutes to ensure complete dissolution. A 50 mM Tris (trimethylolaminomethane) buffer was prepared for addition by dissolving 6.06 grams of tris in 800 mL of water. The solution was titrated to pH 8.0 with 0.1M hydrochloric acid. Additional water was added to dilute the obtained solution to 1 liter. Add 200 mL of Tris buffer to the poloxamer / deoxycholic acid solution. Stir thoroughly to combine the solution.
[0110] Add 20 g of itraconazole and 120 mL of N-methyl-2-pyrrolidone to a 150 mL beaker. Heat the mixture to 50°C-60°C, stirring to dissolve the solids. After complete dissolution was visible, stir for an additional 15 minutes to ens...
Embodiment 2
[0117] Example 2: Preparation of itraconazole suspension using ultrasonication.
[0118] Add 252mL of water for injection into a 500mL stainless steel container. The liquid was heated to 60-65°C, then 6.6 grams of Pluronic F-68 (poloxamer 188), and 0.9 grams of sodium deoxycholate were added slowly, stirring after each addition to dissolve the solids. After complete addition of the solid phase, stir at 60-65°C for 15 minutes to ensure complete dissolution. A 50 mM tris (trishydroxymethylaminomethane) buffer was prepared by dissolving 6.06 g of tris in 800 mL of water for injection. The solution was titrated to pH 8.0 with 0.1M hydrochloric acid. Dilute the obtained solution to 1 liter with additional water for injection. Add 30 mL of tris buffer to the poloxamer / deoxycholate solution. Stir well to combine the solution.
[0119] Add 3 g of itraconazole and 18 mL of N-methyl-2-pyrrolidone to a 30 mL container. Heat the mixture to 50°C-60°C, stirring to dissolve the solid...
Embodiment 3
[0122] Example 3: Preparation of itraconazole suspension by homogenization.
[0123] A 50 mM tris (trishydroxymethylaminomethane) buffer was prepared by dissolving 6.06 g of tris in 800 mL of water for injection. The solution was titrated to pH 8.0 with 0.1M hydrochloric acid. The obtained solution was diluted to 1 liter with additional water for injection. Add 1680 mL of water for injection to a 3 L flask. Add 200 mL of tris buffer to 1680 mL of water. Stir well to combine the solution.
[0124] Add 44 g of Pluronic F-68 (poloxamer 188) to a 150 mL beaker, and add 12 g of sodium deoxycholate to 120 mL of N-methyl-2-pyrrolidone. Heat the mixture to 50°C-60°C, stirring to dissolve the solids. After complete dissolution was visible to the naked eye, it was stirred for an additional 15 minutes to ensure complete dissolution. 20 g of itraconazole were added to the solution and stirred until completely dissolved. The itraconazole-NMP solution was cooled to room temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com