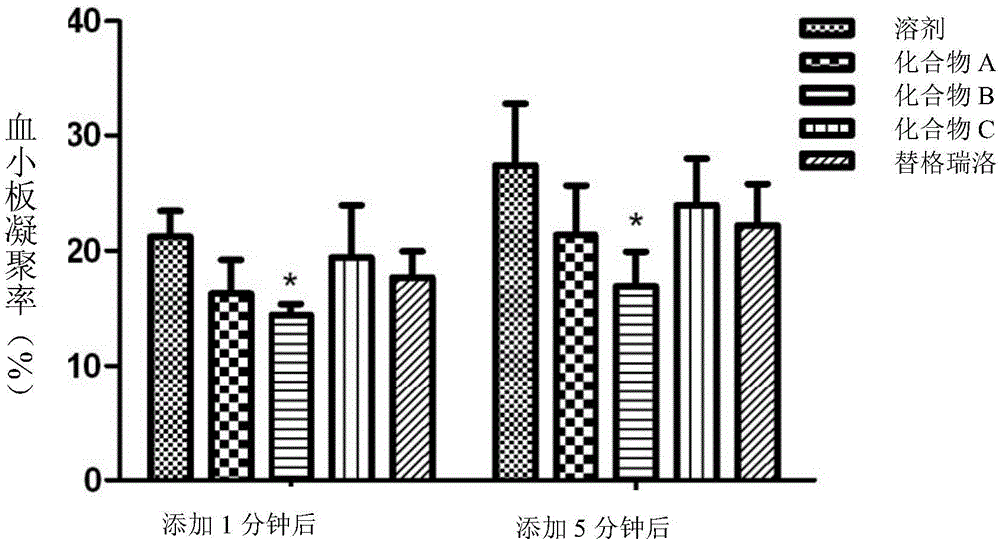
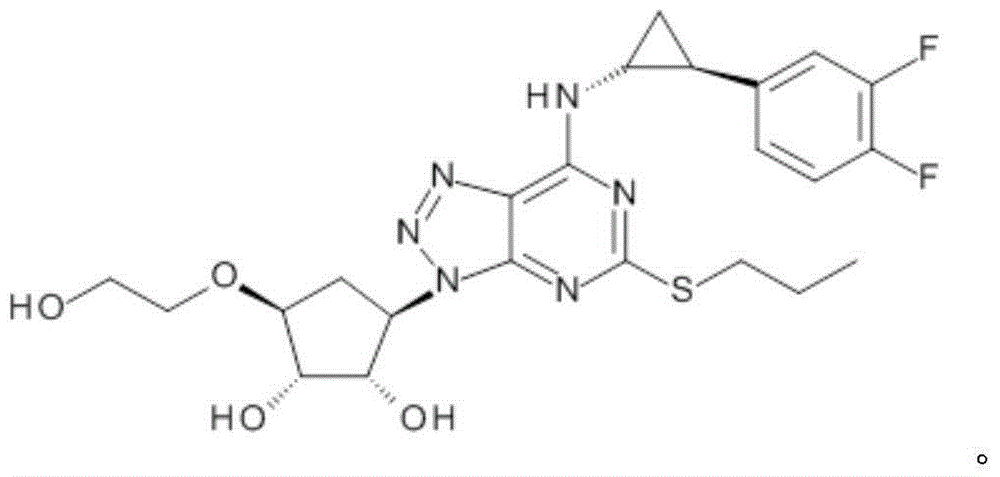
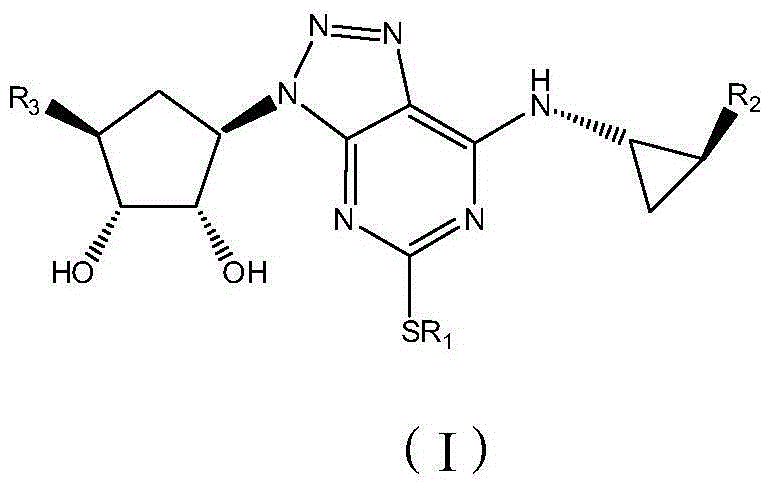
Platelet aggregation inhibitor, and preparation method and application thereof
A solvate and compound technology, applied in the field of medicine, can solve the problem of unsatisfactory anti-platelet aggregation treatment effect and achieve good anti-platelet aggregation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of compound A of the present invention
[0051]
[0052] The concrete preparation method of compound A of the present invention is as follows:
[0053] step 1:
[0054]
[0055]Under a nitrogen inert atmosphere, place (1S,2S,3R,5S)-3-(7-[[(2S)-2-(3,4-difluorophenyl )cyclopropyl]amino]-5-(propylmercapto)-3H-[1,2,3]triazol[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy ) cyclopentane-1,2-diol (2 g, 3.79 mmol, 1.00 equiv, 99%) and 4-methylbenzenesulfonic acid (20 mg, 0.12 mmol, 0.03 equiv, 99%) in acetone (50 mL), Then 2,2-dimethoxypropane (800 mg, 7.68 mmol, 2.03 equiv) was added dropwise with stirring at room temperature. The resulting solution was stirred at 30°C for 12 hours. The pH of the solution was adjusted to 7 with sodium bicarbonate. The resulting mixture was filtered and the filtrate was concentrated in vacuo. The residue was purified by silica gel chromatography and eluted with ethyl acetate:petroleum ether (1:50~1:3) to o...
Embodiment 2
[0067] Embodiment 2: the preparation of compound B of the present invention
[0068]
[0069] The concrete preparation method of compound B of the present invention is as follows:
[0070] step 1:
[0071]
[0072] Under a nitrogen inert atmosphere, place tert-butyl N-[3-[(3aS,4R,6S,6aR)-6-(2-hydroxyethoxy)-2, 2-Dimethyl-hexahydrocyclopenta[d][1,3]dioxol-4-yl]-5-(propylmercapto)-3H-[1,2,3]triazole [4,5-d]pyrimidin-7-yl]-N-[(2S)-2-(3,4-difluorophenyl)cyclopropyl]carbamate (110mg, 0.16mmol, 1.00eq, 99%) and triethylamine (Et 3 N) (35 mg, 0.34 mmol, 2.08 equiv, 99%) in dichloromethane (DCM) (20 mL), then 2-methylbutyryl chloride (28 mg, 0.26 mmol, 1.58 equiv) was added dropwise with stirring. The resulting solution was stirred at 0 °C for 1.5 h, quenched by adding 50 mL of water / ice, and extracted 3 times with 50 mL of dichloromethane. The combined organic layers were dried over anhydrous sodium sulfate, and concentrated under vacuum. The residue was purified on a s...
Embodiment 3
[0078] Embodiment 3: the preparation of compound C of the present invention
[0079]
[0080] The specific preparation method of compound C of the present invention is as follows:
[0081] step 1:
[0082]
[0083] Under a nitrogen inert atmosphere, put tert-butyl N-[3-[(3aS,4R,6S,6aR)-6-(2-hydroxyethoxy)- 2,2-Dimethyl-hexahydrocyclopenta[d][1,3]dioxol-4-yl]-5-(propylmercapto)-3H-[1,2,3] Triazol[4,5-d]pyrimidin-7-yl]-N-[(2S)-2-(3,4-difluorophenyl)cyclopropyl]carbamate (300mg, 0.45mmol, 1.00 Equiv, 99%) in trimethyl phosphate (3 mL), then phosphorus oxychloride (105 mg, 0.68 mmol, 1.51 eq, 99%) was added dropwise. The resulting solution was stirred at 10-15°C for 16 hours, added 50 mL of water / ice to terminate the reaction, and extracted three times with 50 mL of ethyl acetate. The combined organic layers were concentrated in vacuo to afford 200 mg (48%) of (2-[[(3aR,4S,6R,6aS)-6-(7-[[(tert-butoxy)carbonyl][(2S) -2-(3,4-difluorophenyl)cyclopropyl]amino]-5-(propylme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com