Antineoplastic compound, medicament composition and use thereof
A compound and mixture technology, applied in the field of anti-tumor compounds and its preparation, can solve the problems of low solubility, low bioavailability, high side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
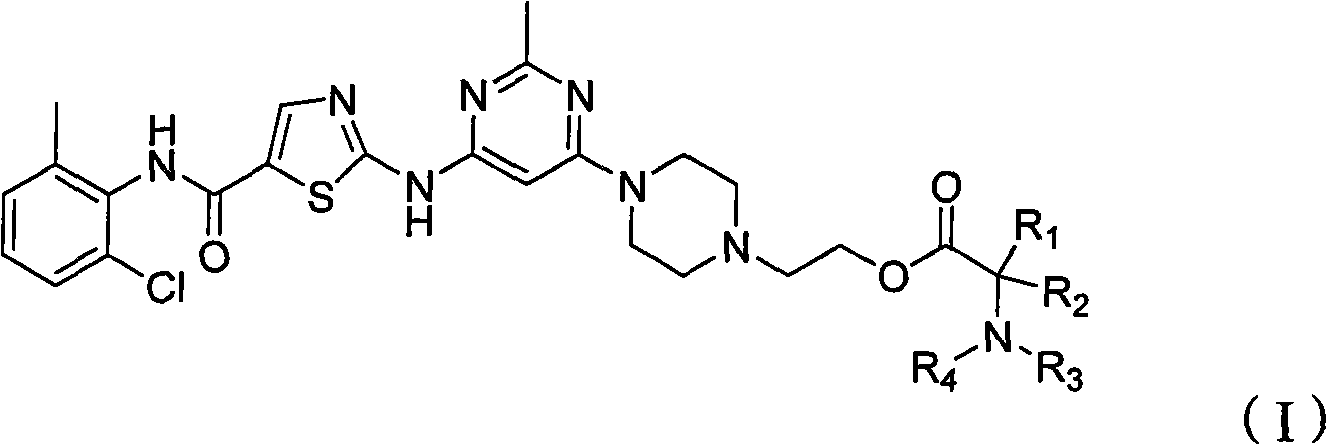
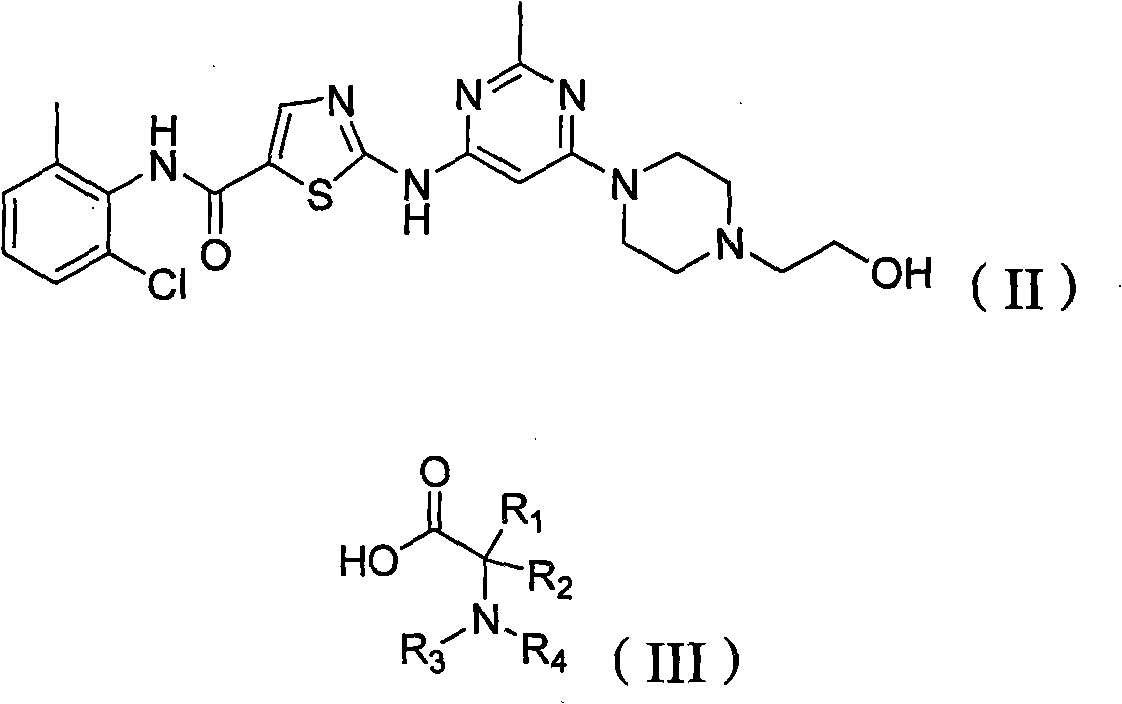
[0239] Example 1: 2-aminopropionic acid (S)-2-(4-(6-(5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-ylamino)-2 -Methylpyrimidin-4-yl)piperazin-1-yl)ethyl ester
[0240] At room temperature, N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidine- 4-ylamino)thiazole-5-carboxamide (487mg, 1.0mmol) was dissolved in anhydrous dimethylformamide (8mL), the solution was placed in an ice bath at 0°C and N-(tert-butoxy Carbonyl) L-alanine (567mg, 3.0mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (582mg, 3.05mmol), N-hydroxybenzotri Azole (411 mg, 3.05 mmol) and N-methylmorpholine (303 mg, 3.0 mmol). The reaction mixture was stirred at 0°C for 30 minutes and at room temperature for 20 hours. The above reaction mixture was diluted with water (150 mL), and extracted with ethyl acetate (200 mL). The organic extract was washed successively with aqueous sodium bicarbonate solution (5%), water and saturated sodium chloride solution, dri...
Embodiment 2
[0242] Example 2: 2-amino-4-methylpentanoic acid (S)-2-(4-(6-(5-((2-chloro-6-methylphenyl)carbamoyl)thiazole-2- Amino)-2-methylpyrimidin-4-yl)piperazin-1-yl)ethyl ester
[0243] Prepared as described in Example 1 except that N-(tert-butoxycarbonyl)L-leucine was used in place of N-(tert-butoxycarbonyl)L-alanine. The resulting compound is C 28 h 37 ClN 8 o 3 S, the calculated value of MS-ESI (m / z) is 600.2; the measured value is 601.5 (100, MH + ), 603.0 (40, (MH +2 ) + ).
Embodiment 3
[0244] Example 3: 2-Aminoacetic acid (S)-2-(4-(6-(5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-ylamino)-2- Methylpyrimidin-4-yl)piperazin-1-yl)ethyl ester
[0245] Prepared as described in Example 1 except that N-(tert-butoxycarbonyl)L-glycine was used in place of N-(tert-butoxycarbonyl)L-alanine. The resulting compound is C 24 h 29 ClN 8 o 3 S, the calculated value of MS-ESI (m / z) is 544.2; the measured value is 545.3 (100, MH + ), 546.9 (40, (MH +2 ) + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com