Polymorph of SYK inhibitors
A polymorphic, dimesylate technology, applied in anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve problems such as different pharmacodynamic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0315] Example 1: Synthesis of polymorph 3
[0316] General methods for preparing the different forms of the compound of formula I can be found in US Patent Nos. 8,450,321 and 8,455,493. The following is a process for the preparation of polymorph 3, which is the polymorph of the dimesylate salt monohydrate of the compound of formula I (and can also be described as the monohydrate of the compound of formula IA shown in the following embodiments. polymorphic form).
[0317]
[0318] To Reactor A was charged compound of Formula I (1.0X). To Reactor B was added methanesulfonic acid (0.56X, 2.40eq), water (4X, 4V) and acetone (3.2X, 4V). The contents of Reactor B were added to Reactor A while maintaining the temperature in Reactor A below 35°C. After the solids had dissolved, the contents of Reactor A were transferred to Reactor B. Reactor A was rinsed with water (1X, 1V) and acetone (0.8X, 1V) and transferred to Reactor B. The temperature of Reactor B was adjusted to 19-25...
Embodiment 2
[0320] Embodiment 2: another synthesis method of polymorph 3
[0321] The following is a method for preparing polymorph 3 of the dimesylate salt monohydrate of the compound of formula I (which may also be described as a polymorph of the monohydrate of the compound of formula IA shown in the following reaction schemes).
[0322]
[0323] Polymorph 7 was obtained as described in Example 1.
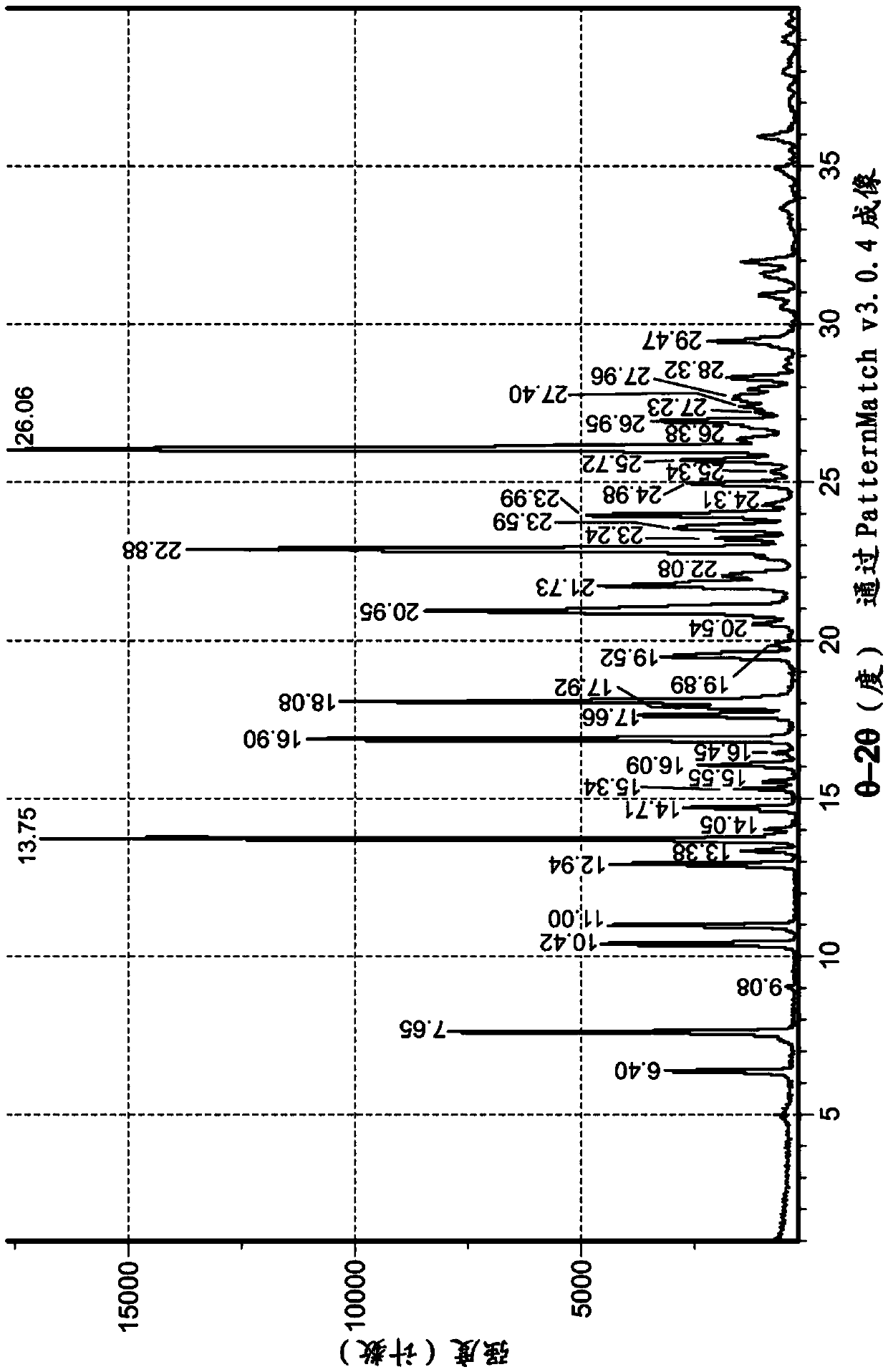
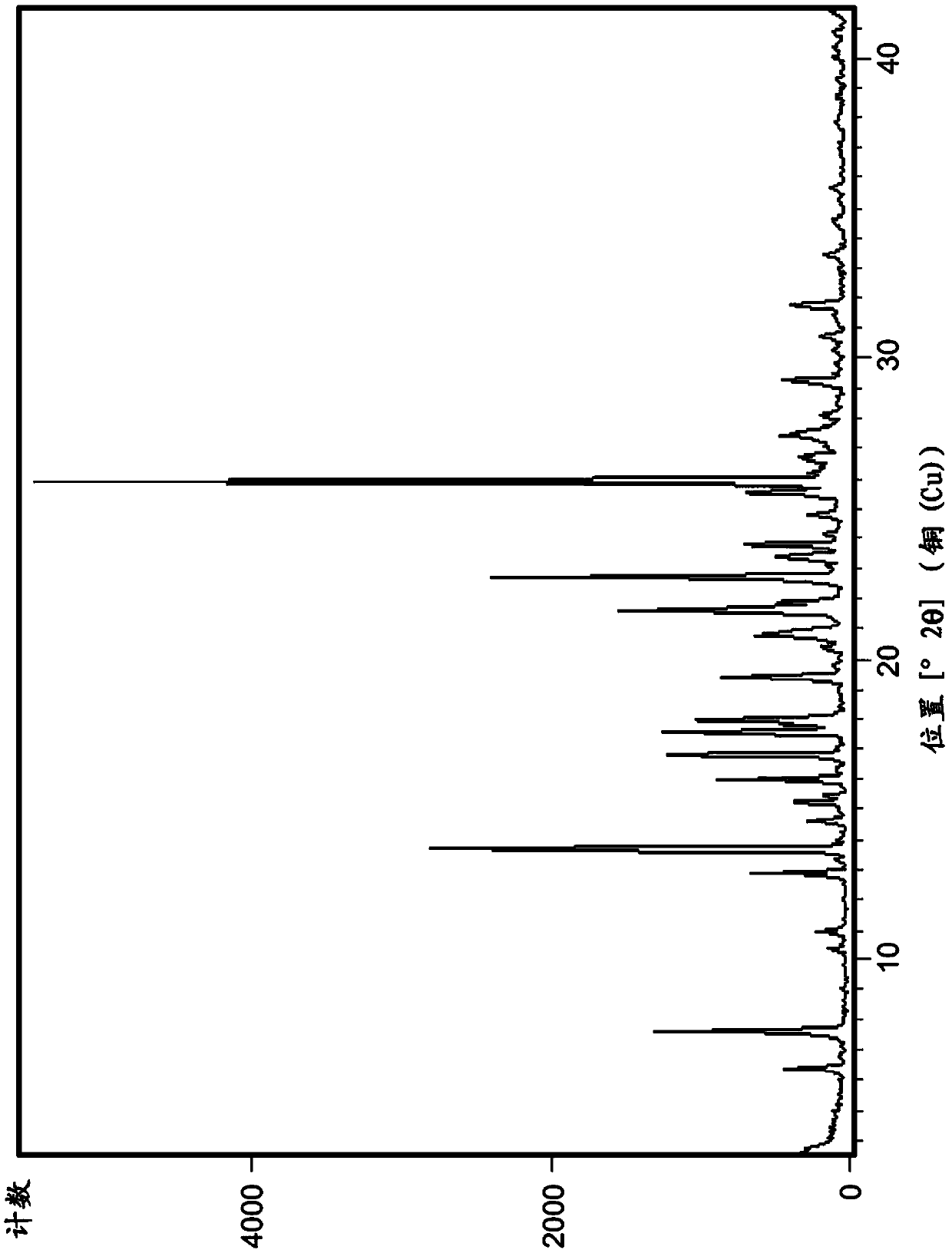
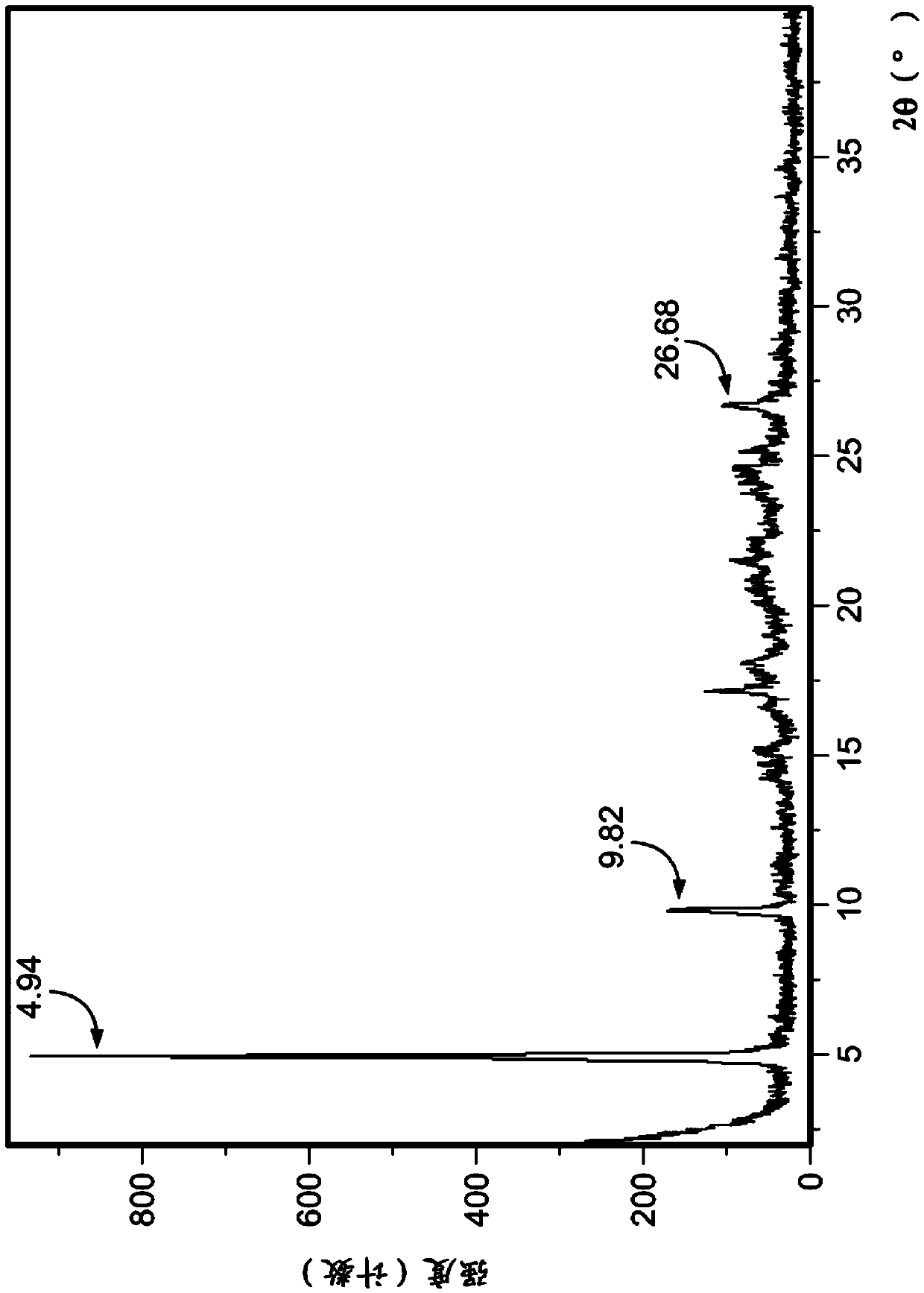
[0324] The isolated polymorph 7 was seeded in reactor B with polymorph 3 of the compound of formula IA (0.01X, 1 mol%). Acetone (15.0X, 19.0V) and water (1.0X, 1.0V) were added to Reactor B. The contents of Reactor B were heated to reflux (about 55°C) until polymorph Form 7 was converted to Form 3. The conversion was monitored by XRPD or DSC. The contents of Reactor B were a slurry and were cooled to 19-25°C, then filtered, rinsed with acetone (2.4X, 3V) and dried in vacuo at 60°C until constant weight was reached to provide polymorph 3 . exist Figure 1A and 3A Representative XRPD ...
Embodiment 3
[0325] Example 3: One-pot synthesis of polymorph 3
[0326] The following is the preparation of polymorph 3 of the dimesylate salt monohydrate of the compound of formula I (which can also be described as the monohydrate of the compound of formula IA shown in the reaction scheme below) from the compound of formula I (as the free base) polymorphic form) method. The process uses a single reactor in the conversion of the compound of formula I to polymorph 3 and does not require the isolation of compound intermediates.
[0327]
[0328] Compound of Formula I (1.0X) was added to acetone (14.2X, 18V) in Reactor A and mixed. Water (0.8X, 0.8V) was charged to Reactor A, followed by methanesulfonic acid (0.48X, 2.05eq). Acetone (0.9X, 1.2V) was pumped in, flushing the tubing to Reactor A with acetone. The contents of Reactor A were heated to reflux (about 55°C) for about 2 hours. Reactor A was seeded with polymorph 3 (0.015X, 1 mol%) and the contents mixed at reflux to convert th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com