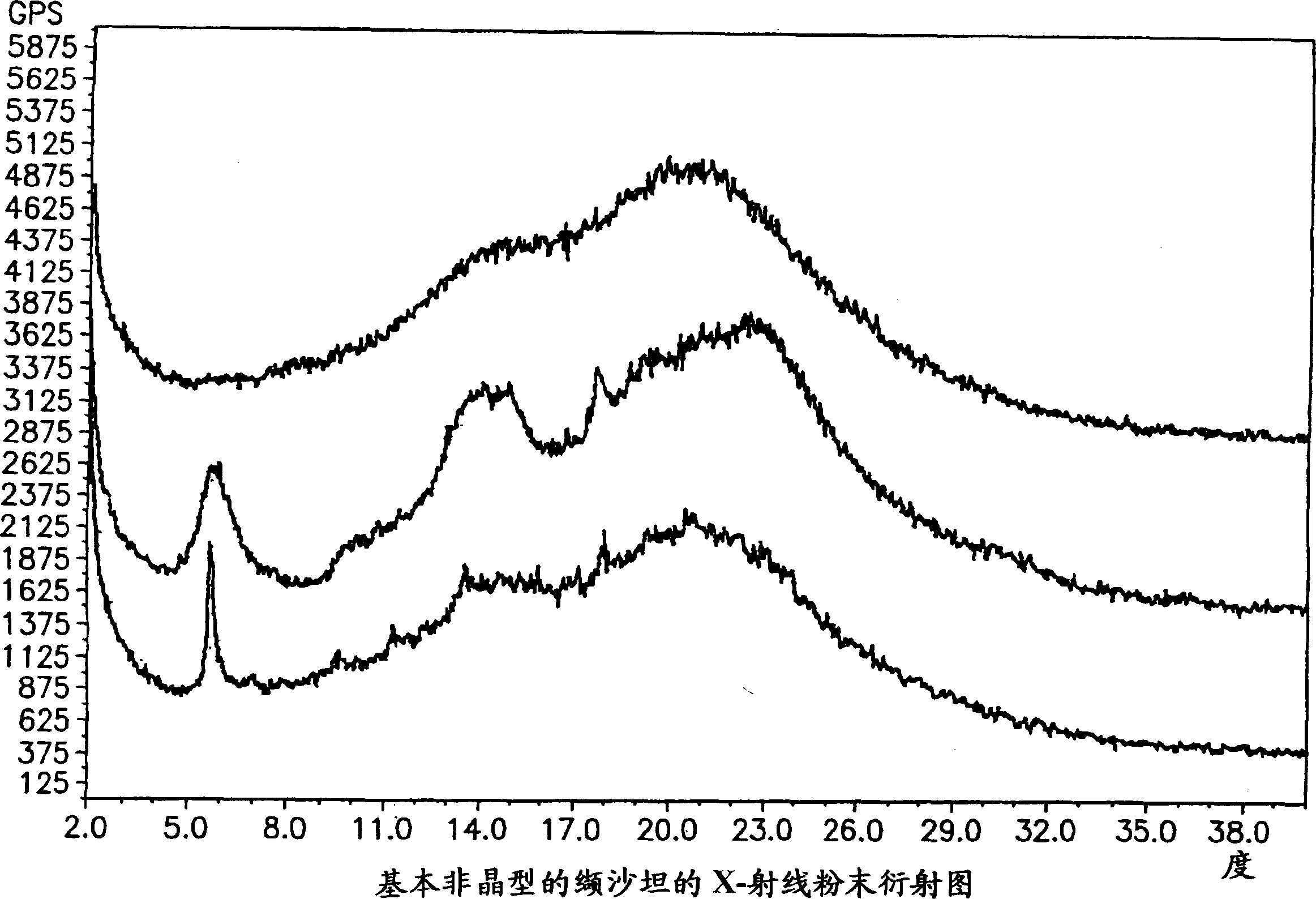
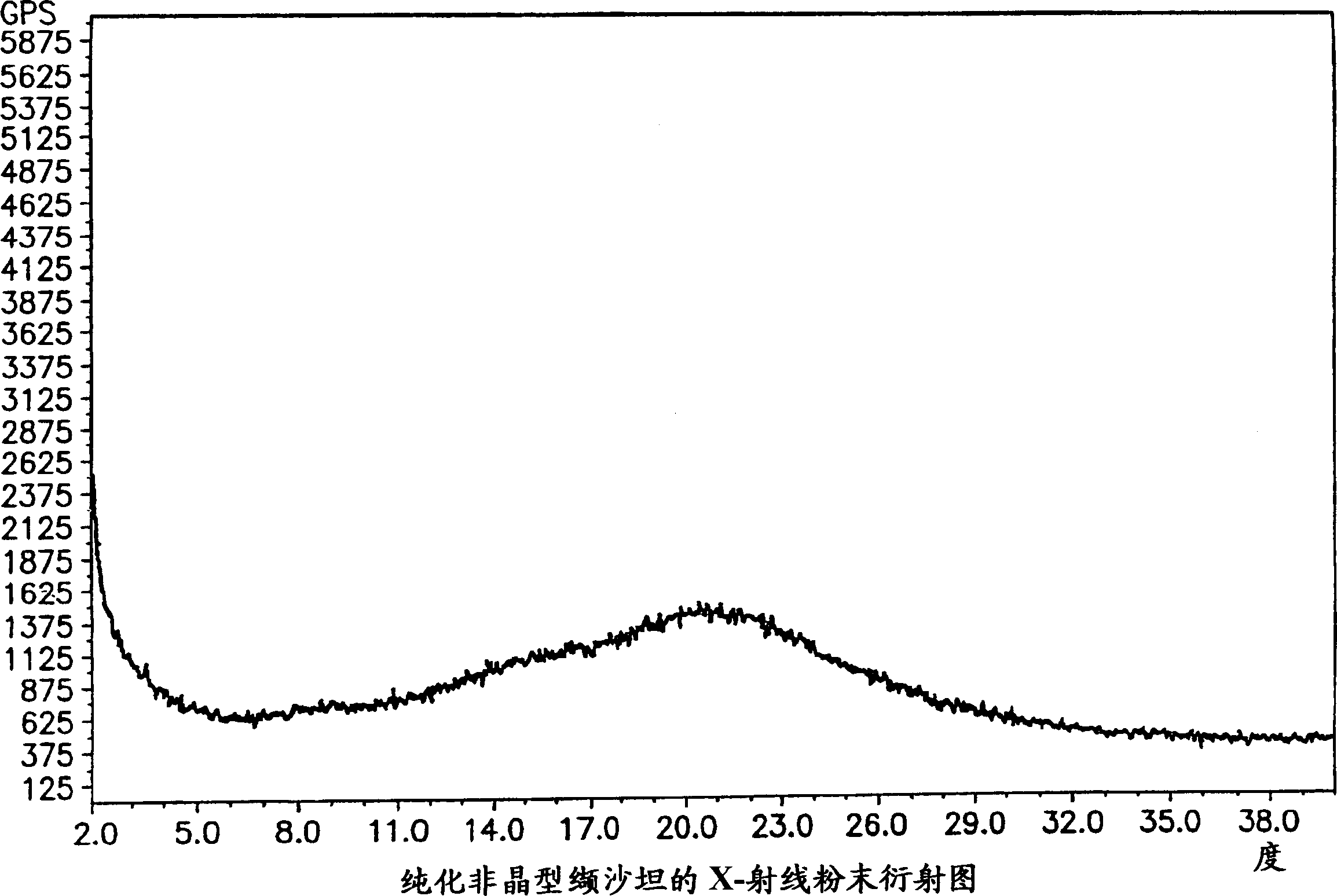
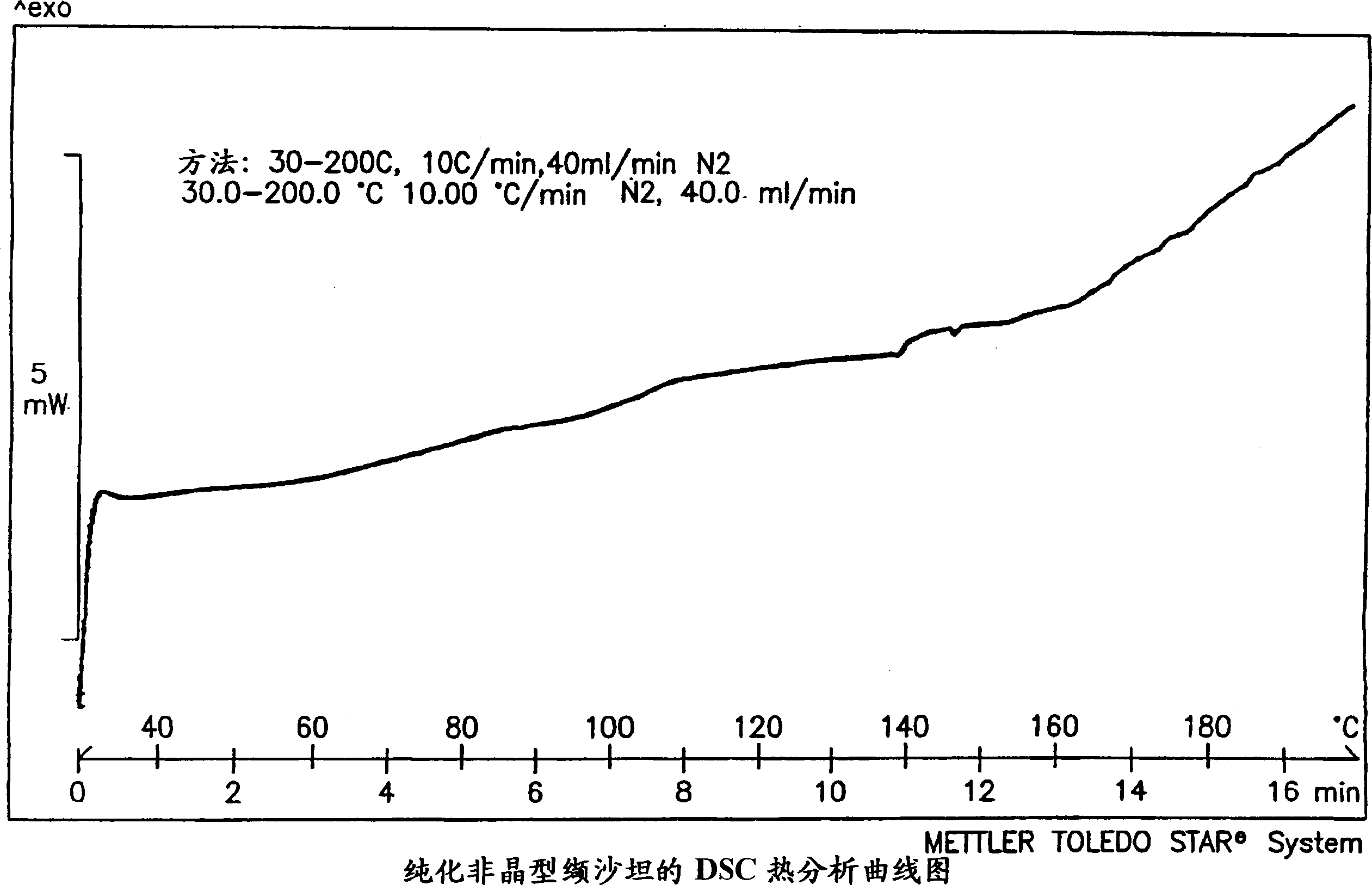
Polymorphis of valsartan
A technology for valsartan and tan crystal forms, applied in the field of solid-state chemistry of valsartan, can solve problems such as not describing product characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] from tert-butyl acetate
[0166] 2 g of valsartan was dissolved in 15 ml of refluxing t-BuOAc to form a solution. The solution was cooled to room temperature with slow stirring, cooled to 0 °C and allowed to stand for 2 hours. The precipitate was filtered and left on the filter for 10 minutes. X-ray analysis showed that the sample had Form III crystals. The sample was dried at 50° C. / 10 mmHg for 2 hours, X-ray analysis showed that the dried sample was a purified amorphous form, and an endothermic peak was detectable in DSC.
Embodiment 2
[0168] from methyl tert-butyl ether
[0169] 5 g of valsartan were dissolved in 20 ml of refluxing MTBE, and the resulting solution was cooled to room temperature with slow stirring, then to 0° C. and allowed to stand for 3 hours. The precipitate was filtered, held on the filter for 10 minutes and dried at 50°C / 10 mmHg for 2 hours. X-ray analysis showed the sample to be a purified amorphous form and no endothermic peak was detected in DSC.
Embodiment 3
[0171] from isoethyl ether
[0172] Dissolve 2g valsartan in 35ml i-Pr 2 O, under reflux for 1 hour, most of the valsartan forms a viscous gelatinous residue. The solvent was decanted and the residue was dried at 50°C / 10 mmHg for 2 hours. X-ray analysis showed the sample to be a purified amorphous form and no endothermic peak was detected in DSC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com