BTK inhibitor polymorph and preparation method thereof
A polymorph, crystal form technology, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problem of different binding force of crystalline particles, affecting drug dissolution rate, affecting drug fluidity, particle uniformity, content uniformity and physical stability. issues of sex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1, (R, E)-5-amino-1-(1-(4-methoxybut-2-enonyl)pyrrol-3-yl)-3-(4-phenoxyphenyl ) Preparation of -1H-pyrazole-4-amide polymorph A
[0041] The compound (R, E)-5-amino-1-(1-(4-methoxybut-2-enonyl)pyrrol-3-yl)-3-(4-phenoxyphenyl)- 1H-Pyrazole-4-amide (55.0g), ethyl acetate (200mL) and dichloromethane (10mL) were added to a 1000mL single-necked flask, heated to 85°C, all solids were dissolved, filtered while hot, and the filtrate was added to Put it into another 1000mL single-necked flask, cool to room temperature, slowly stir and crystallize, filter, and vacuum dry at 50°C overnight to obtain 40.8g of off-white solid, yield: 74.2%.
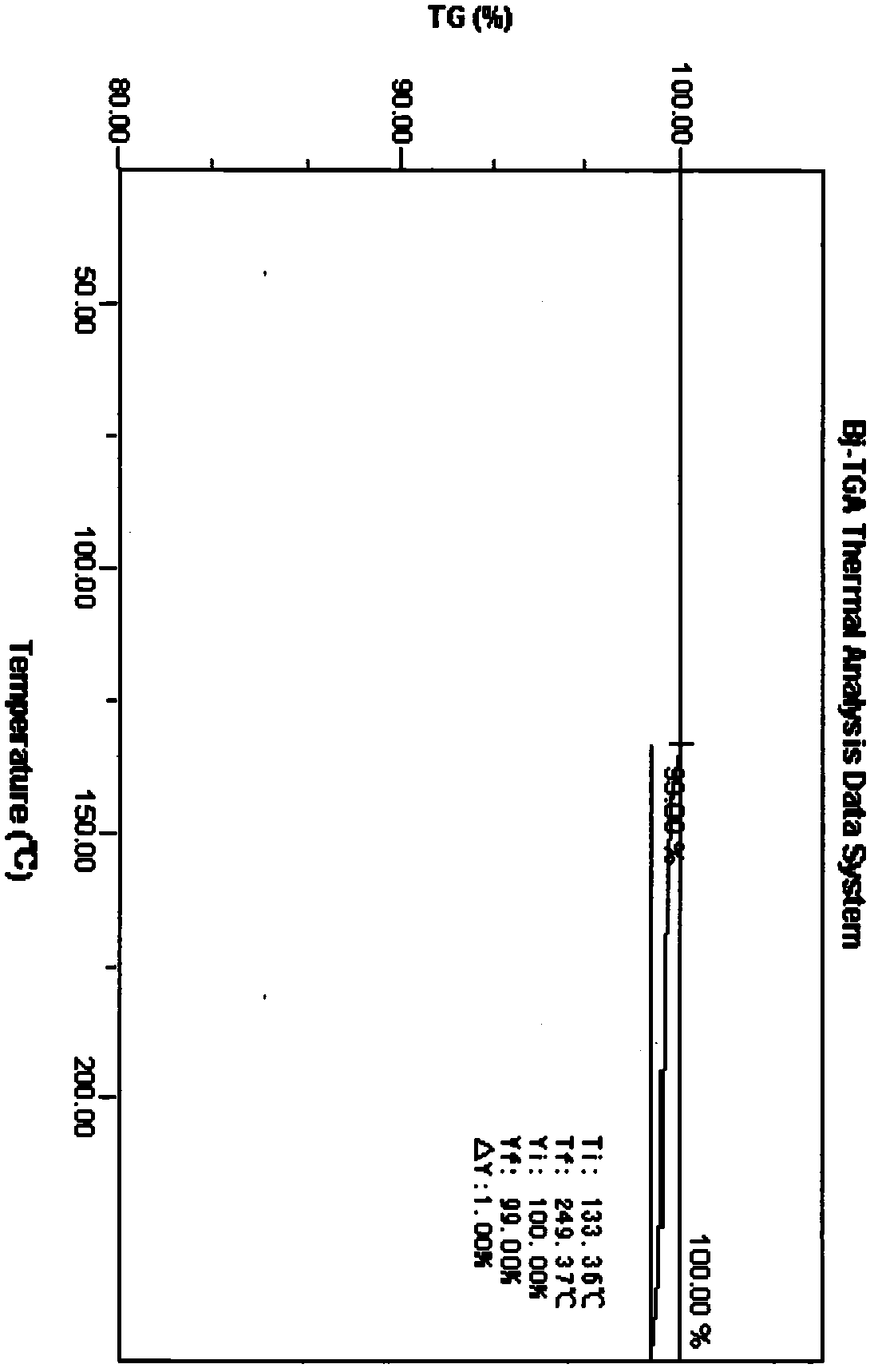
[0042] DSC detection chart (initial temperature 25°C, end temperature 250°C, heating rate 10°C / min) such as Image 6 shown.
Embodiment 2
[0043]Example 2, (R, E)-5-amino-1-(1-(4-methoxybut-2-enonyl)pyrrol-3-yl)-3-(4-phenoxyphenyl )-1H-pyrazole-4-amide polymorph B preparation
[0044] The compound (R, E)-5-amino-1-(1-(4-methoxybut-2-enonyl)pyrrol-3-yl)-3-(4-phenoxyphenyl)- 1H-pyrazole-4-amide (polymorphic form A: 10.5g) and purified water (20mL) were added to a 50mL single-necked flask, heated to 50°C, stirred for 4 days, suction filtered, and vacuum-dried overnight at 50°C. 10.2 g of off-white solid was obtained, yield: 97.0%.
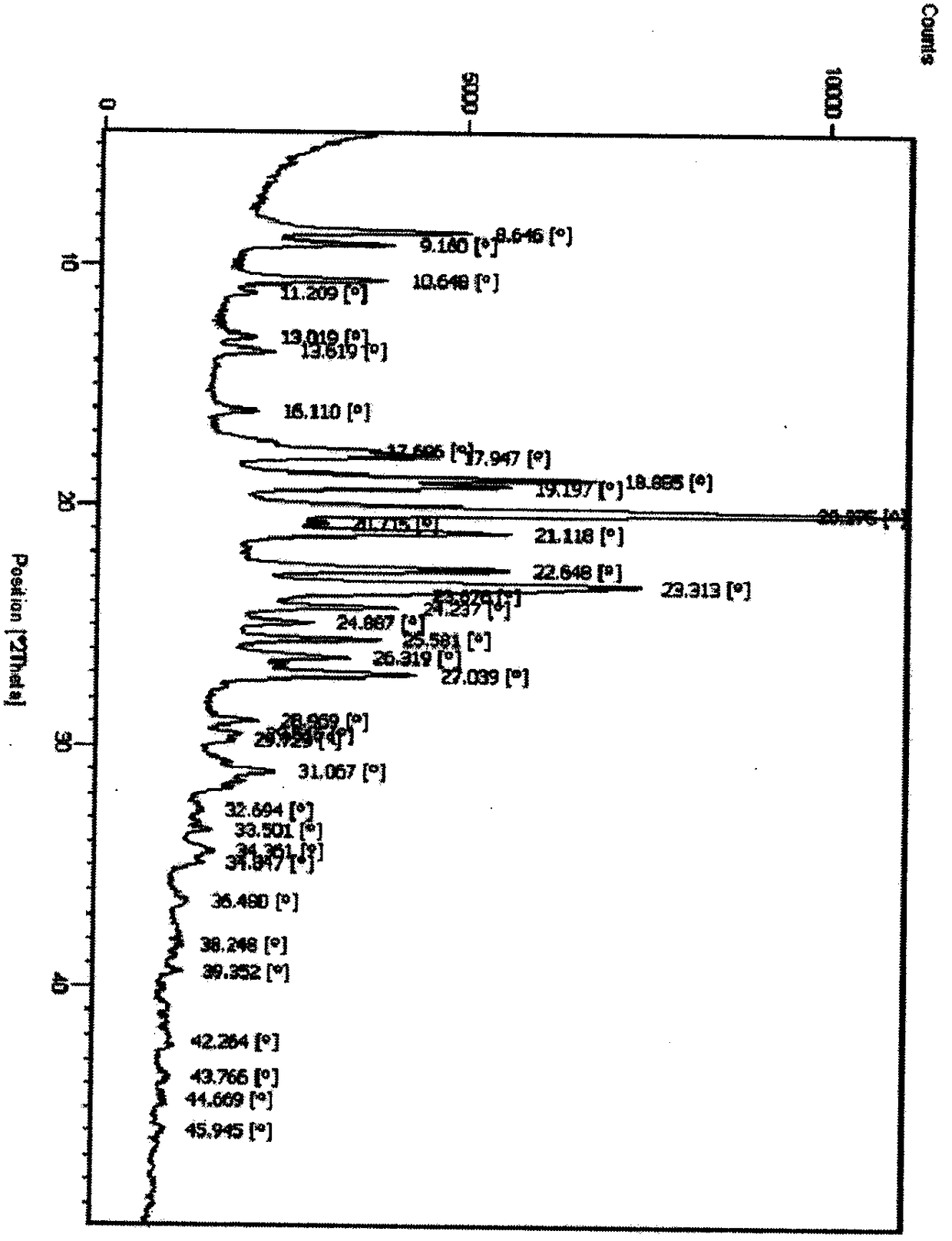
[0045] DSC detection chart (initial temperature 25°C, end temperature 250°C, heating rate 10°C / min)) such as Figure 7 shown.
Embodiment 3
[0046] Example 3, (R, E)-5-amino-1-(1-(4-methoxybut-2-enonyl)pyrrol-3-yl)-3-(4-phenoxyphenyl ) Preparation of -1H-pyrazole-4-amide polymorph C
[0047] The compound (R, E)-5-amino-1-(1-(4-methoxybut-2-enonyl)pyrrol-3-yl)-3-(4-phenoxyphenyl)- 1H-Pyrazole-4-amide (15.0g) and ethyl acetate (100mL) were added to a 500mL single-necked flask, heated to reflux, all the solids were dissolved, filtered while hot, and the filtrate was added to another 500mL single-necked flask , cooled to room temperature, slowly stirred for crystallization, filtered, and vacuum-dried at 50° C. overnight to obtain 13.5 g of off-white solid, yield: 90.0%.
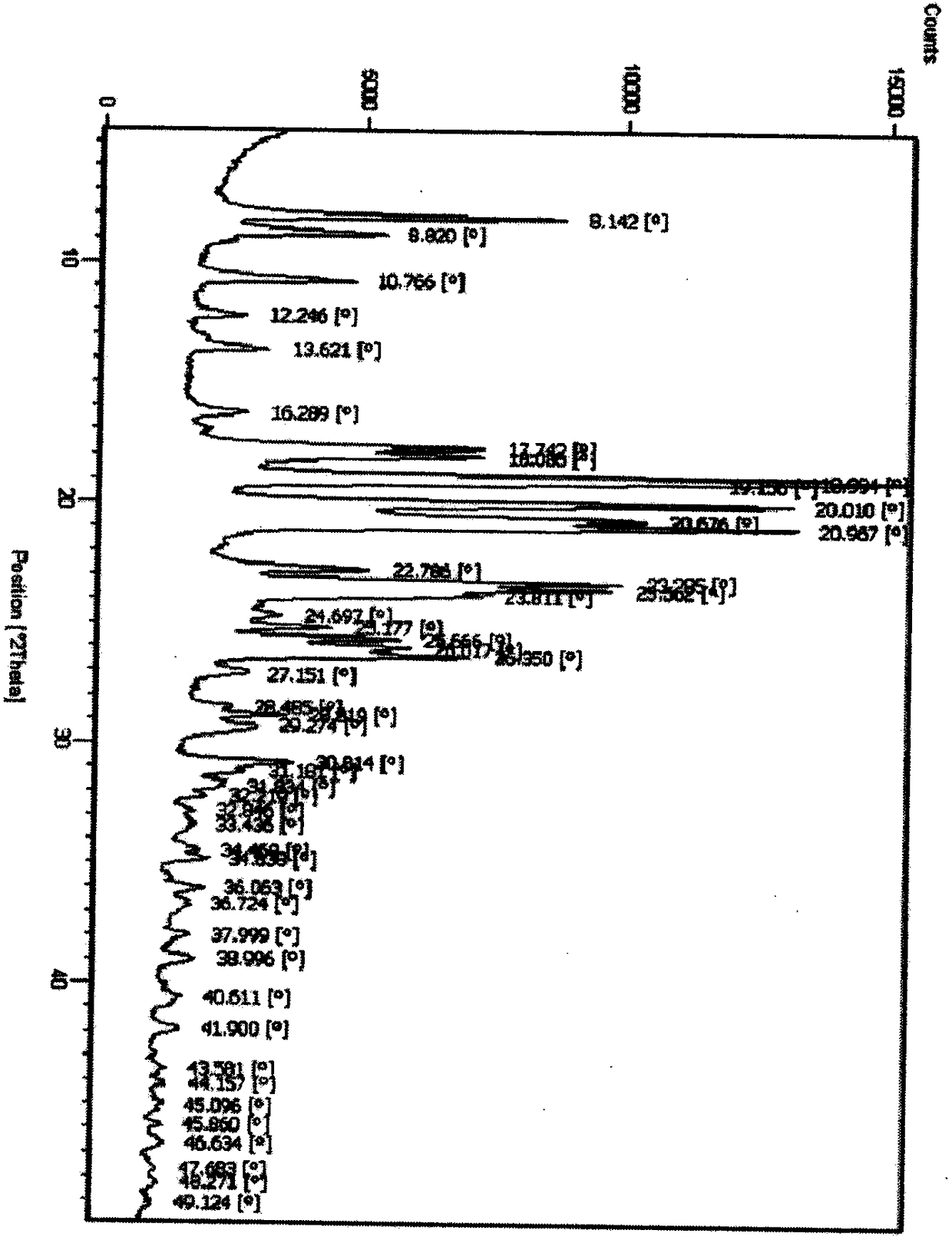
[0048] DSC detection chart (initial temperature 25°C, end temperature 250°C, heating rate 10°C / min) such as Figure 8 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com