Patents
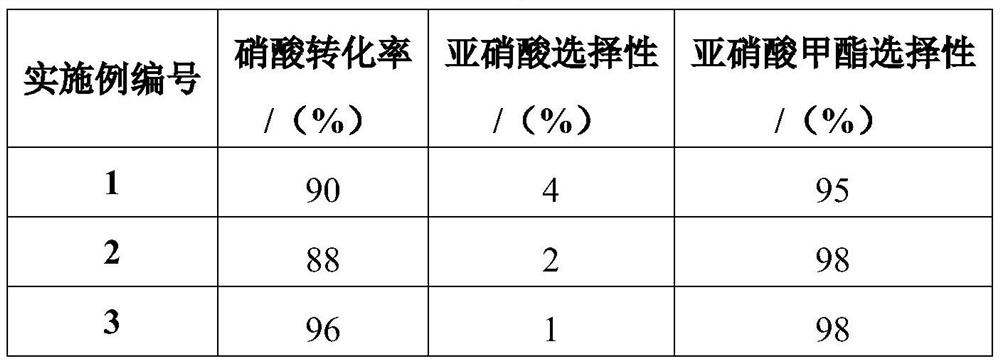
Literature
36 results about "Methyl nitrate" patented technology
Efficacy Topic
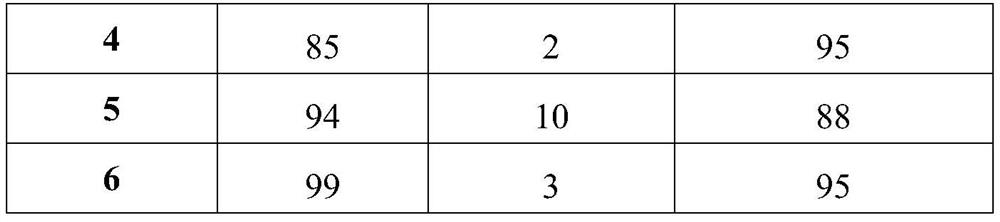
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methyl nitrate is the methyl ester of nitric acid and has the chemical formula CH₃NO₃. It is a colourless volatile liquid that is explosive.
Environmental protection and energy saving methanol diesel
InactiveCN1431280ANo pollution in the processSimple processLiquid carbonaceous fuelsEngineeringMethyl nitrate
An environment protection type energy-saving methanol diesel oil is prepared from diesel oil, methanol, C12, methyl nitrate, and castor oil. Its advantage is no environmental pollution.
Owner:李宇翔
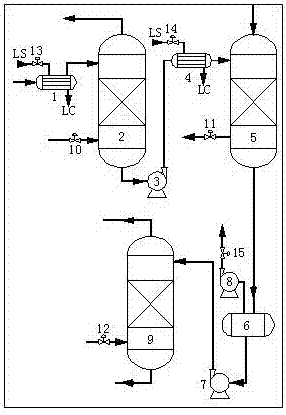
Method and equipment for continuously producing methyl nitrite
InactiveCN102898311AReduce generationInvestment minimizationNitrous acid preparation ester preparationMethyl nitrateOxygen
The invention relates to a method and equipment for continuously producing methyl nitrite in large scale. The method for producing methyl nitrite comprises the following steps of: carrying out the esterification of methanol, oxygen and a nitrogen oxide in an esterification reaction tower to obtain methyl nitrite; circulating and returning the liquid flow from the outlet at the bottom of the esterification reaction tower to the top of the tower, leading the gas flow from the outlet at the bottom of the esterification reaction tower to a purification tower and washing the gas from the bottom of the esterification tower by countercurrent contact with an organic purifier; drying the gas from the top of the purification tower in a dryer to obtain methyl-nitrite-containing dry gas; and leading liquid at the bottom of the purification tower to a recovery tower, and separating a recovery purifier. The methyl nitrite produced by the method can be directly used to prepare dimethyl oxalate through oxidization, carbonylation and coupling reaction with CO. Compared with the conventional production process, the method for producing methyl nitrite has the characteristics of economy, environment friendliness, less waste water and the like.
Owner:SHANGHAI HUAYI ENERGY CHEM
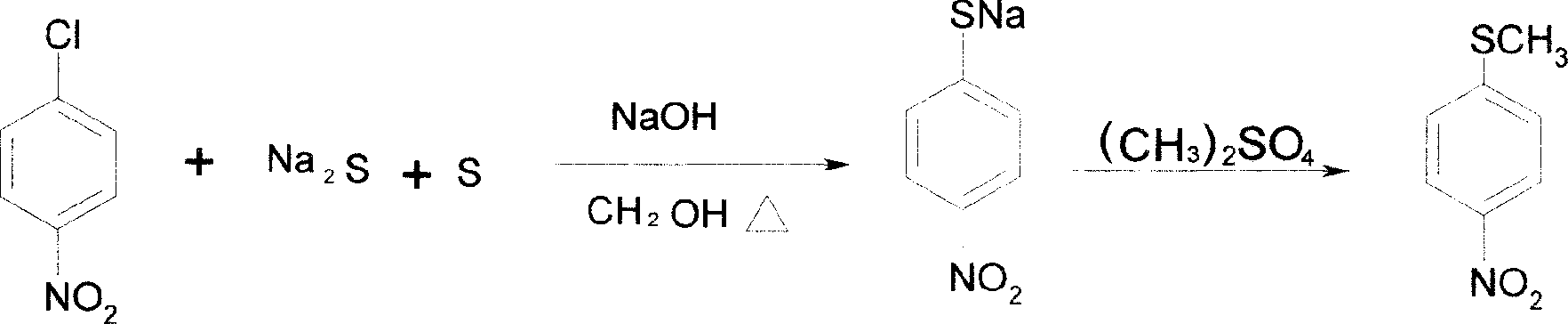
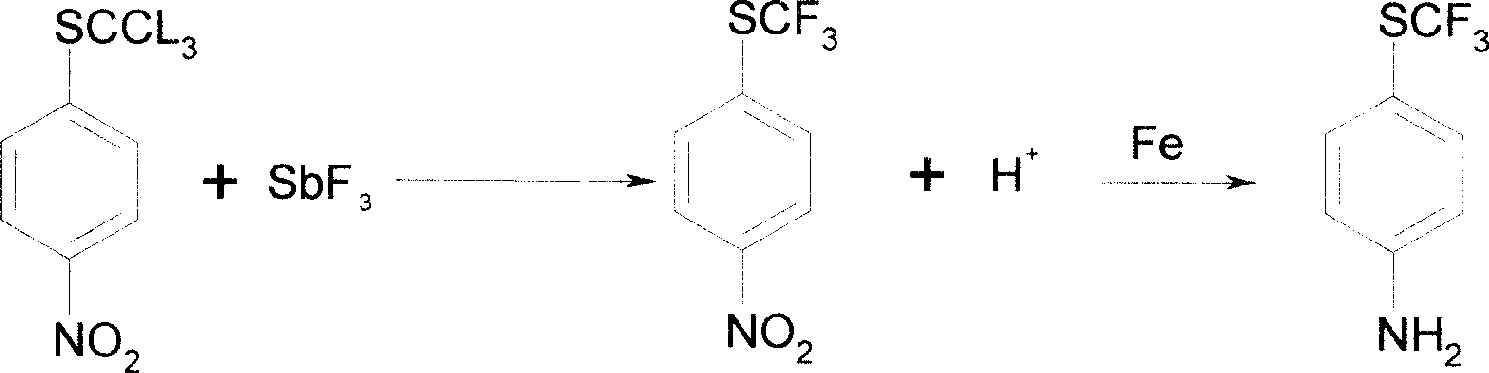
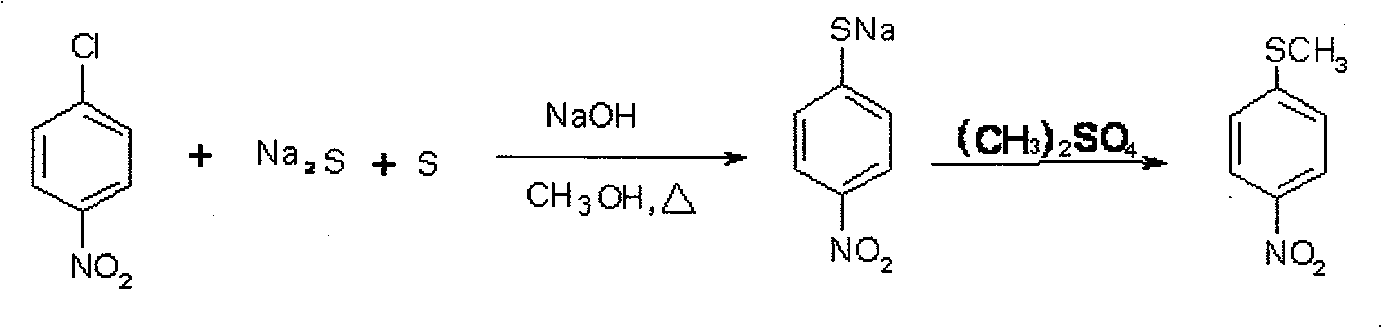
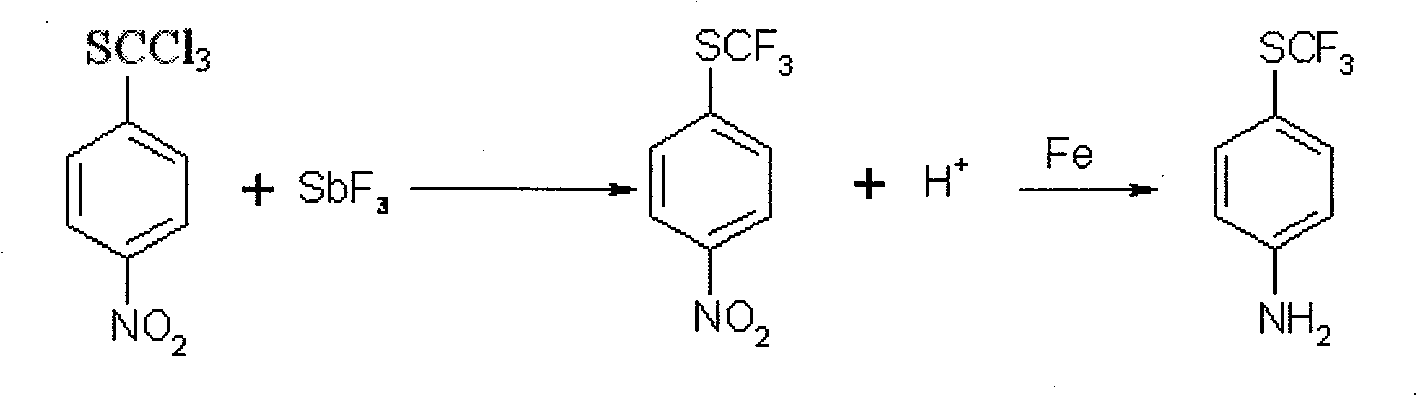
Method of producing toltrazuril
A preparation method of the Toltrazuril is provided. The Toltrazuril is the 1-[3-methyl-4-(4- Trifluoromethylthiobenzoxy) benzyl]-3-methyl-1, 3, 5, -triazine-2, 4, 6(1H, 3H, 5H)-trione. The methyl sulfide chloro compounds, fluoro-compounds, amides, phenol, methyl nitrate chlorobenzene, benzene aether, ammonia benzene aether, isonitrile acid ester and methyl urea from the reactions of 4-nitrochlorobenzene, sulfur, sodium sulfide and dimethyl sulfate. The detailed preparation is that reaction 1: the methanol of 410g and 4-nitrochlorobenzene of 158g are mixed and heated until soluble and are added with sulfur, sodium sulfide and methanol mixed liquor by drops, and kept for 2h under temperature of 60 DEG C. to 65DEG C.; reduce the temperature, add water of 880g and add dimethyl sulfate of 192g; during the period, the pH value of the sodium hydroxide is adjusted over 9, and the methyl sulfide of 153.6 is gained by filtering after reaction with yield of 91.1 per cent.
Owner:PU LIKE BIO ENG +1
Regeneration process and regeneration device system of methyl nitrite in process for preparation of ethylene glycol from synthesis gas
PendingCN106588667ASolve the problem of dissolving methyl nitriteReduce consumptionNitrous acid preparation ester preparationNitrogen oxideMethyl nitrate
The invention relates to a regeneration process and a regeneration device system of methyl nitrite in a process for preparation of ethylene glycol from a synthesis gas, the regeneration process comprises the following steps: (1) methyl nitrite regeneration, to be more specific, a liquid containing methanol and nitric acid and a gas containing nitrogen oxides from a methyl nitrite synthesis reactor are introduced into a methyl nitrite regeneration tower for reaction to obtain methyl nitrite; and (2) methyl nitrite stripping recovery, to be more specific, a methyl nitrite regeneration tower kettle components and a gas or gases selected from one or more of N2, CO2, CH4, CO and the nitrogen oxides are introduced into a methyl nitrite regeneration stripping tower for stripping for recovery of the methyl nitrite dissolved in the methanol. The regeneration process reduces the consumption of the nitric acid in the process for preparation of the ethylene glycol from the synthesis gas, the nitric acid produced by side reaction is economically and efficiently recovered, and the problem that a methanol solution dissolves the methyl nitrite can be solved.
Owner:SHANGHAI HUAYI ENERGY CHEM
System and method for recovering methyl nitrite through coal ethylene glycol carbonyl synthesis system
InactiveCN107056582AReduce shortingReduce pollutionOrganic compound preparationHydroxy compound separation/purificationChemical reactionFluid phase
The invention relates to the field of coal ethylene glycol chemical engineering and particularly relates to a system and method for recovering methyl nitrite through a coal ethylene glycol carbonyl synthesis system. According to the method, the main target of effectively recovering a methyl nitrite gas is achieved in the coal ethylene glycol carbonyl synthesis system through the methods of combining air stripping, flash evaporation and nitric acid reduction. The recovery system mainly comprises a front air stripping system for methyl nitrite, a nitric acid reduction system and a rear air stripping system, wherein the front air stripping system is provided with a liquid-phase preheater, a front air stripping tower and a liquid-phase discharge centrifugal pump; the nitric acid reduction system is provided with a liquid-phase feeding preheater and a nitric acid reduction tower; and the rear air stripping system is provided with the flash evaporation tank, the liquid-phase feeding centrifugal pump and the rear air stripping tower. Stable content of nitric oxide and the methyl nitrite in a gas-phase component of the synthesis system is ensured through the method of combining a physical process and chemical reaction, the losses of the nitric oxide and the methyl nitrite are avoided to a great extent, the waste is changed into treasure, and resource waste and water source pollution are reduced.
Owner:安阳永金化工有限公司
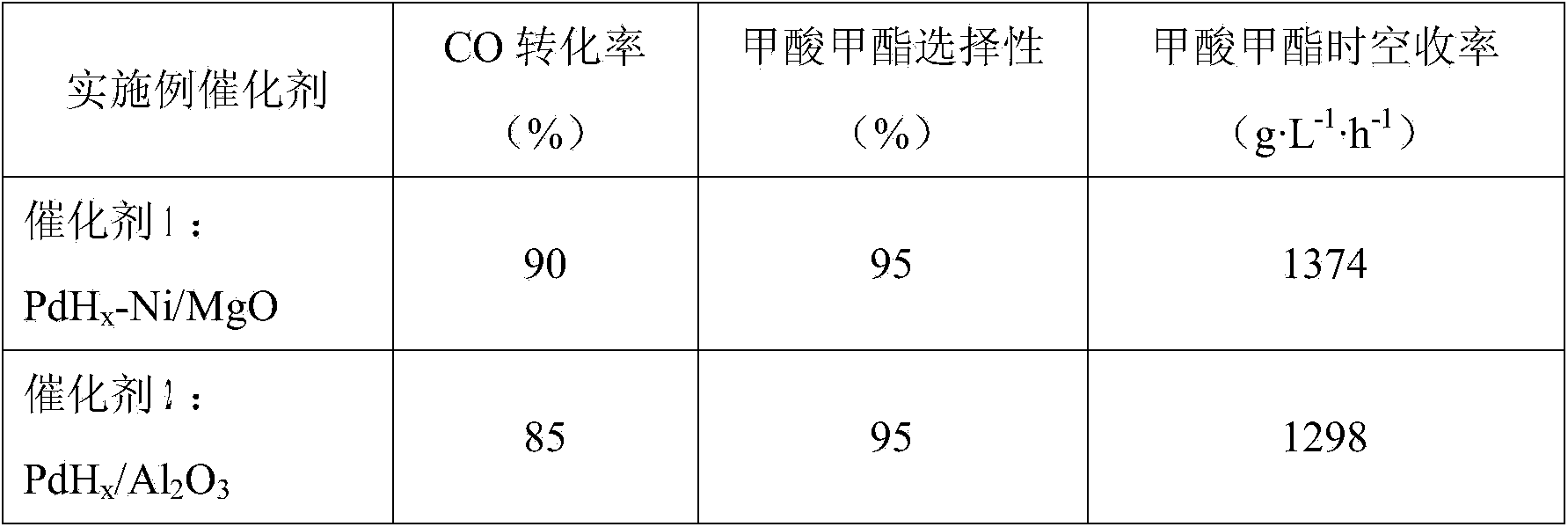
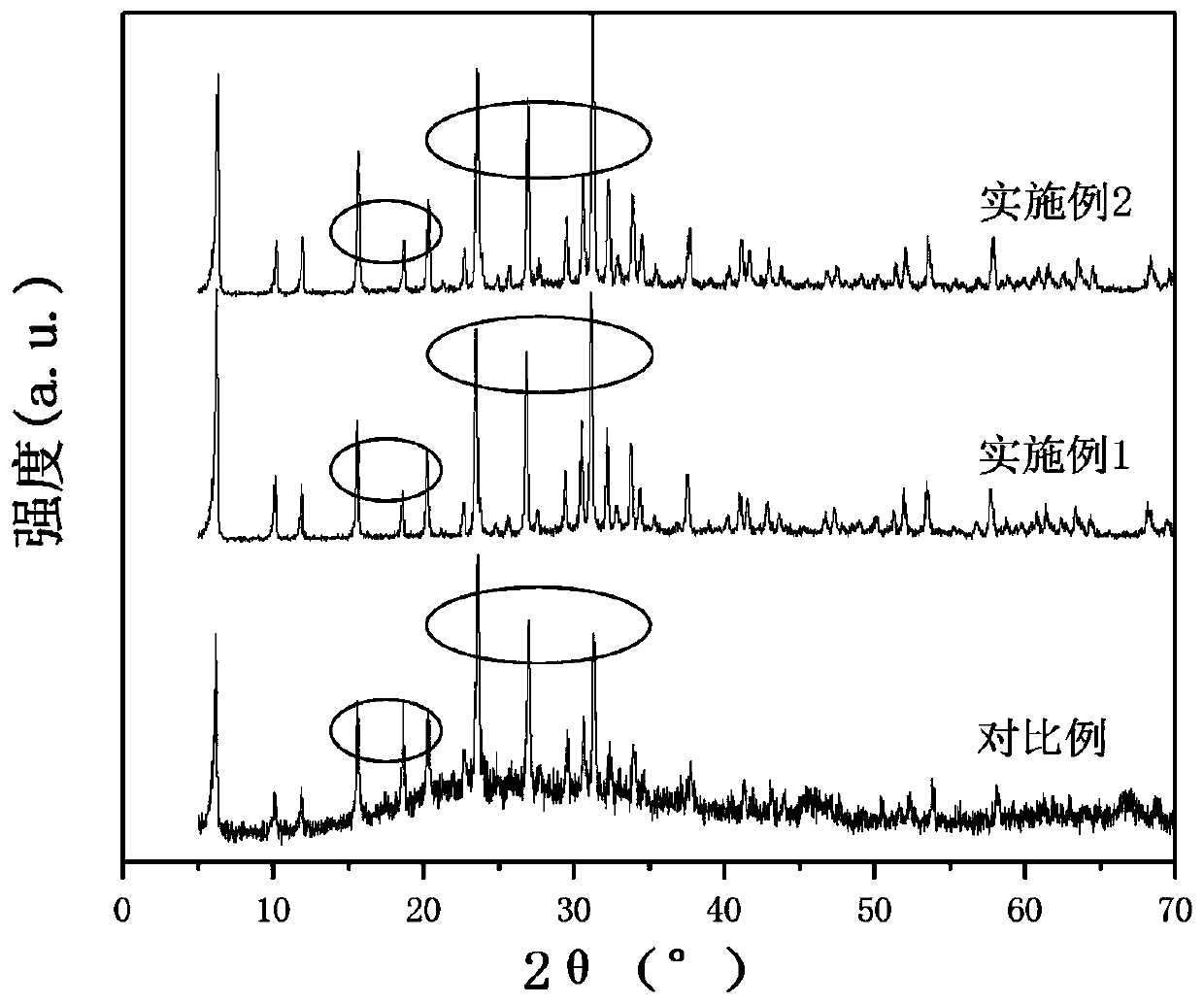
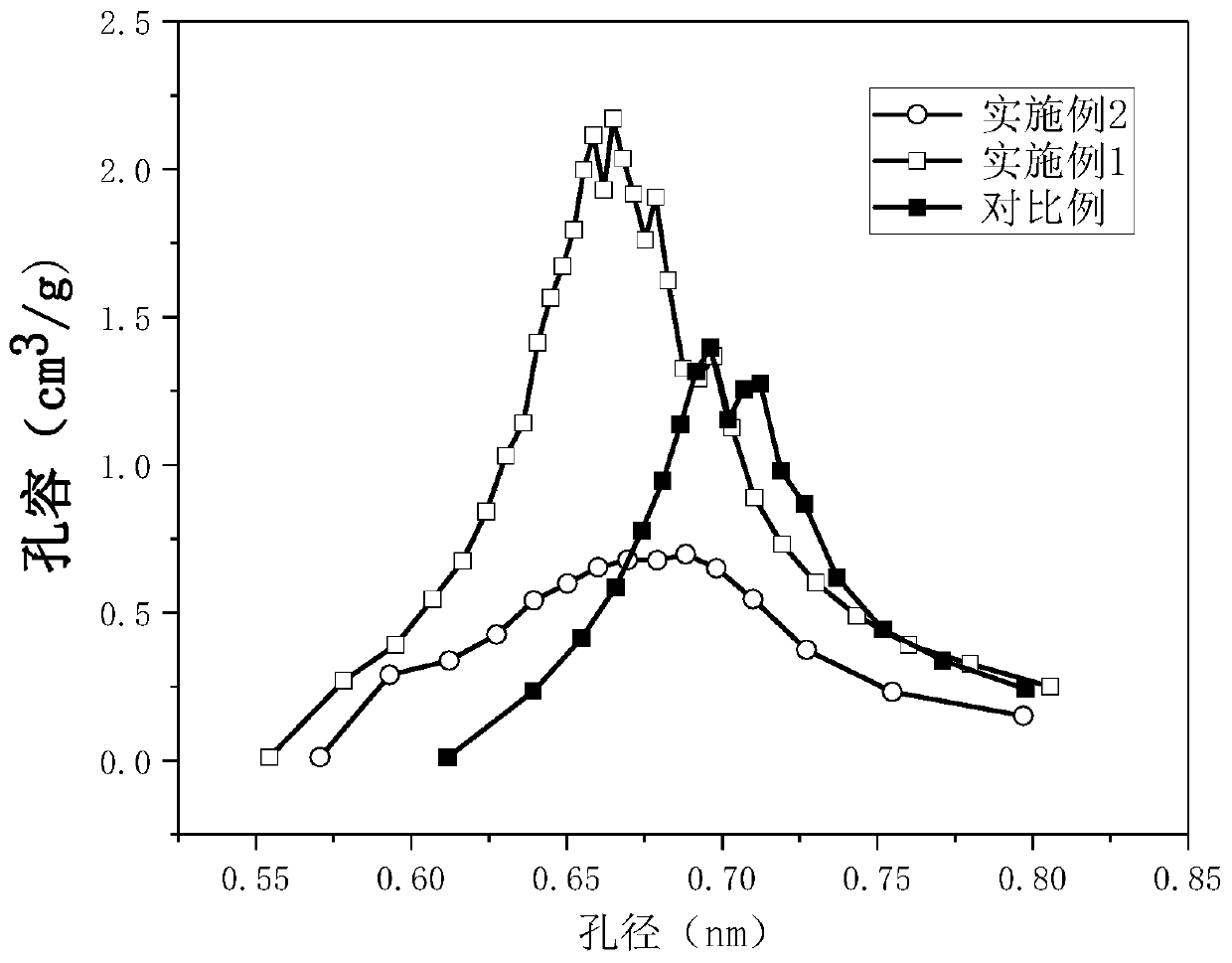
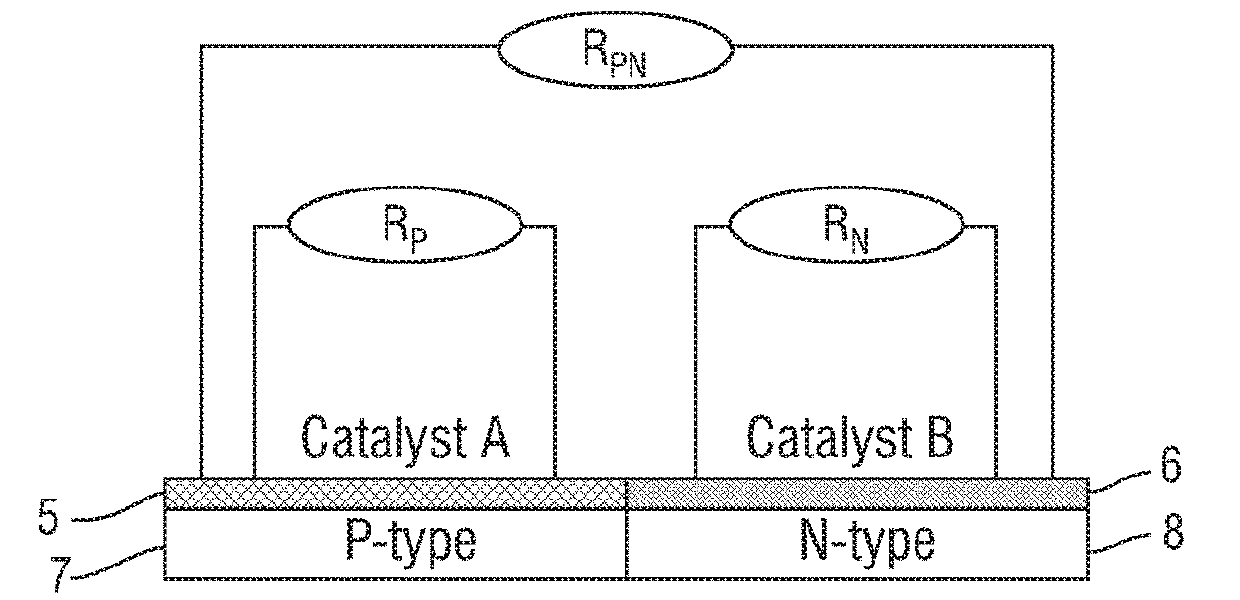
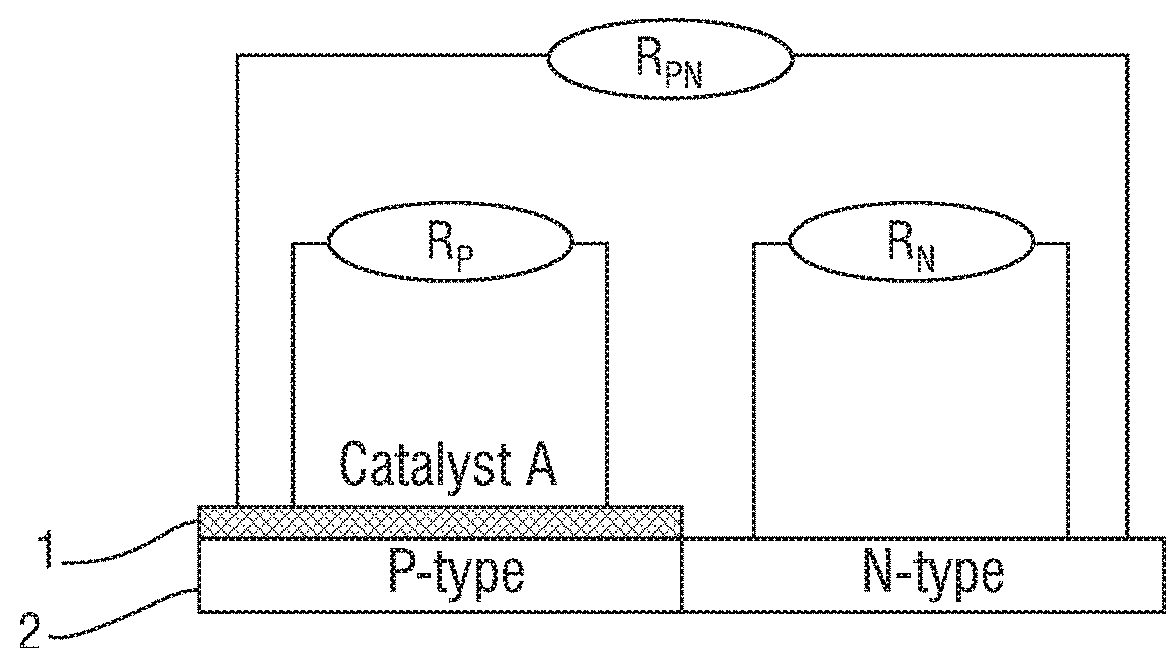
Catalyst for synthesizing methyl formate by formylating methyl nitrite, preparation method and application of catalyst
ActiveCN103894232AImprove stabilityLow impurity content requirementOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide or formate reactionCyanideHydrogen
The invention discloses a supported catalyst applicable to reaction for synthesizing methyl formate by formylating methyl nitrite. The supported catalyst comprises a palladium cyanide active component, a carrier and an optional accessory ingredient. According to the mass of the carrier, the percentage of the active component palladium cyanide is 0.01-2%, and the percentage of the accessory ingredient is less than or equal to 20%. The invention also discloses a preparation method of the catalyst applicable to reaction for synthesizing methyl formate by formylating methyl nitrite. The preparation method comprises the following steps: optionally dipping the carrier into water or ethanol solution of an accessory ingredient precursor, standing, drying and roasting; then dipping the carrier into water or ethanol solution of a palladium precursor, evenly stirring and evenly dispersing the carrier in a palladium precursor solution; carrying out ultrasound treatment under the heating condition until a solvent of the solution is volatilized to be dry, and evenly absorbing the palladium precursor on the surface of the carrier; drying and roasting an obtained absorption sample; and then reducing in the atmosphere of high-purity hydrogen to obtain a supported type palladium cyanide catalyst.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Y molecular sieve for synthesizing dimethyl carbonate through carbonylation of methyl nitrite, and preparation method thereof
ActiveCN110844918AImprove conversion rateHigh space-time yieldCatalyst carriersMolecular sieve catalystsMolecular sievePtru catalyst
The invention discloses a Y molecular sieve for synthesizing dimethyl carbonate through carbonylation of methyl nitrite, and a preparation method thereof, wherein the Y molecular sieve is synthesizedby adopting a hydrothermal method and a microwave-assisted crystallization method. The preparation method comprises: preparing a guiding agent, regulating a mother liquor composition by using a titanium source and a phosphorus source, carrying out microwave pre-crystallization treatment, and carrying out a crystallization reaction to obtain the Y molecular sieve. According to the invention, the Ymolecular sieve is characterized in that titanium and phosphorus heteroatoms are doped into a framework on the basis of keeping of a Y molecular sieve framework structure, and the active component loading molecular sieve is used as a chlorine-free system catalyst for synthesizing dimethyl carbonate through carbonylation of methyl nitrite to achieve good catalytic activity.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
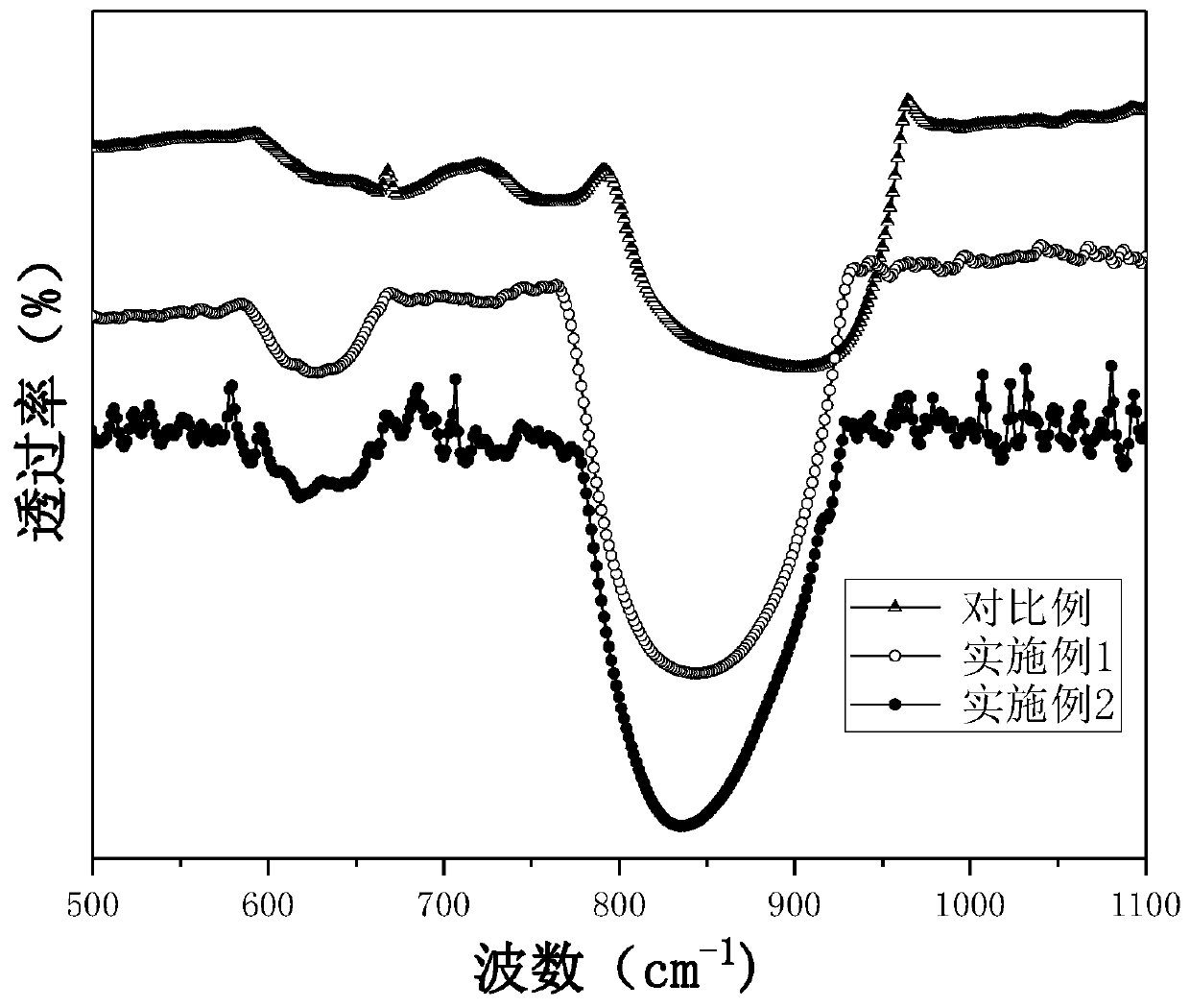
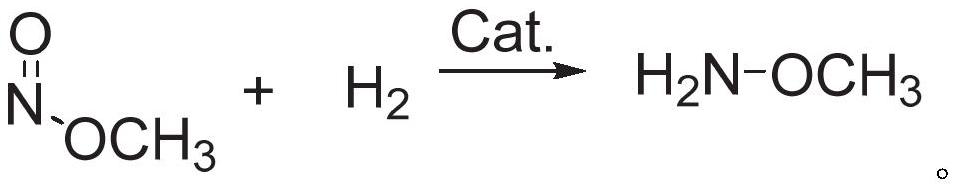
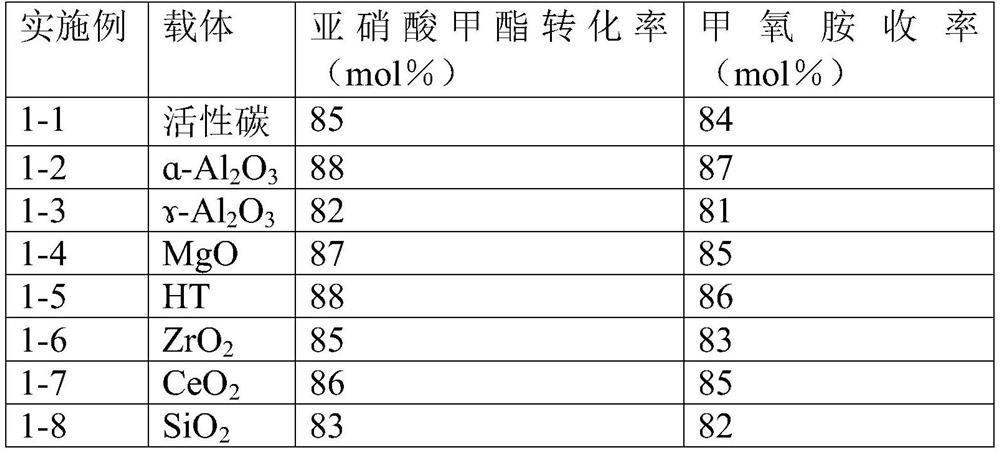
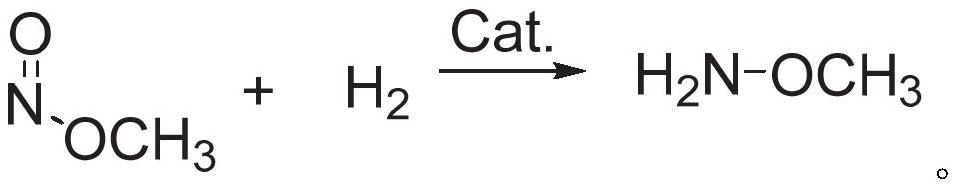
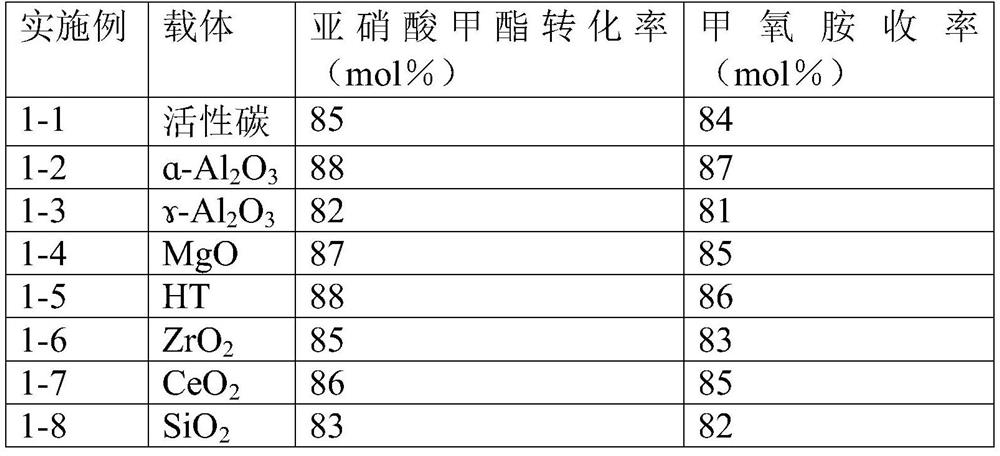
Methoxylamine preparation method and methoxylamine hydrochloride preparation method
ActiveCN112125822AImprove conversion efficiencyEasy to operateOrganic chemistryPtru catalystMethoxylamine
The invention discloses a methoxylamine preparation method which at least comprises the following steps of enabling feed gas containing methyl nitrite and a reducing agent to be in contact with a reduction reaction catalyst in a reactor, and performing reduction reaction to obtain methoxylamine. According to the method, the important intermediate methyl nitrite in the technical process of preparing ethylene glycol from coal can be fully utilized, and the conversion rate of methyl nitrite is high. The invention also provides a method for preparing methoxylamine hydrochloride by taking methoxylamine obtained by the method as a raw material.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
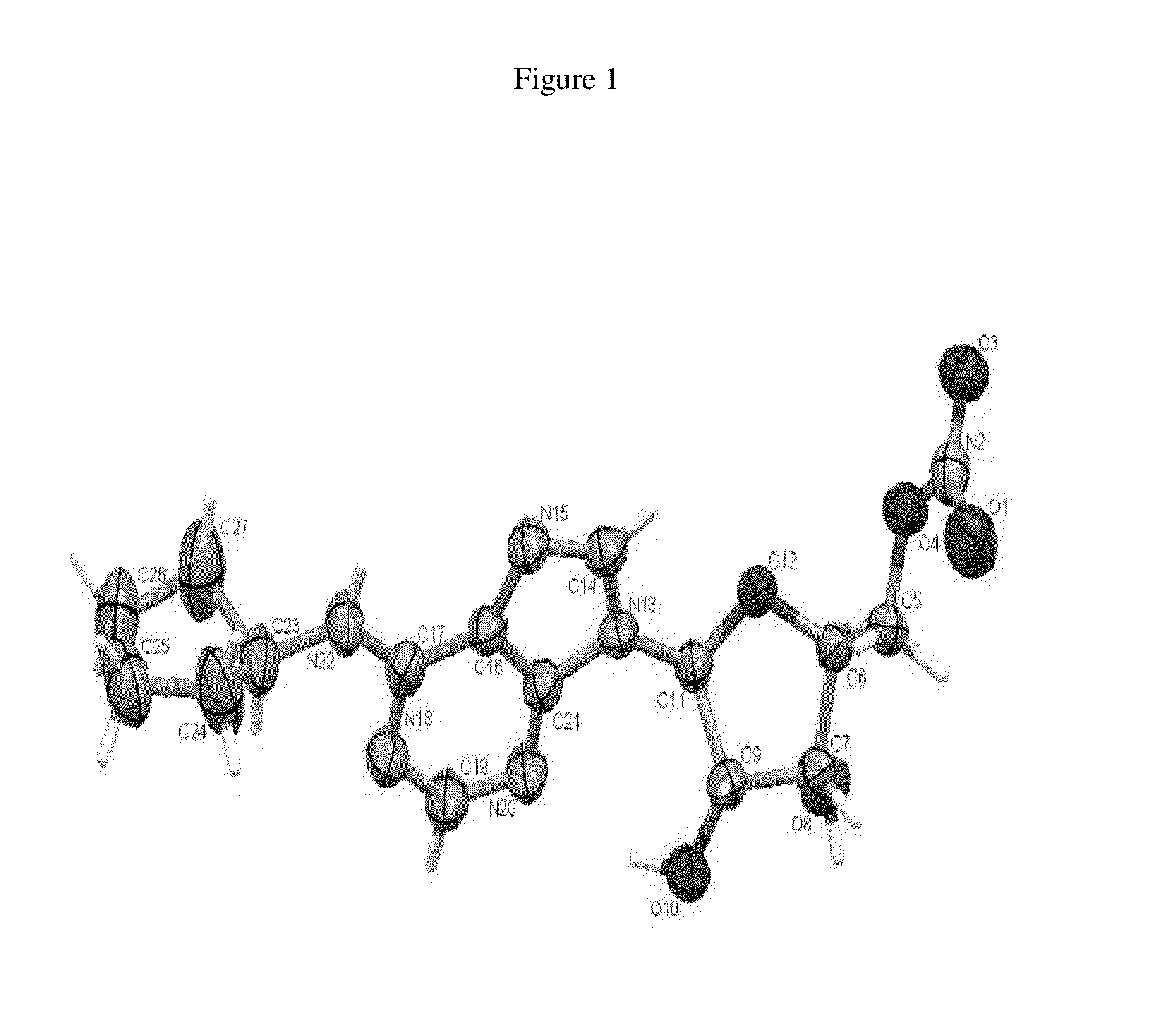
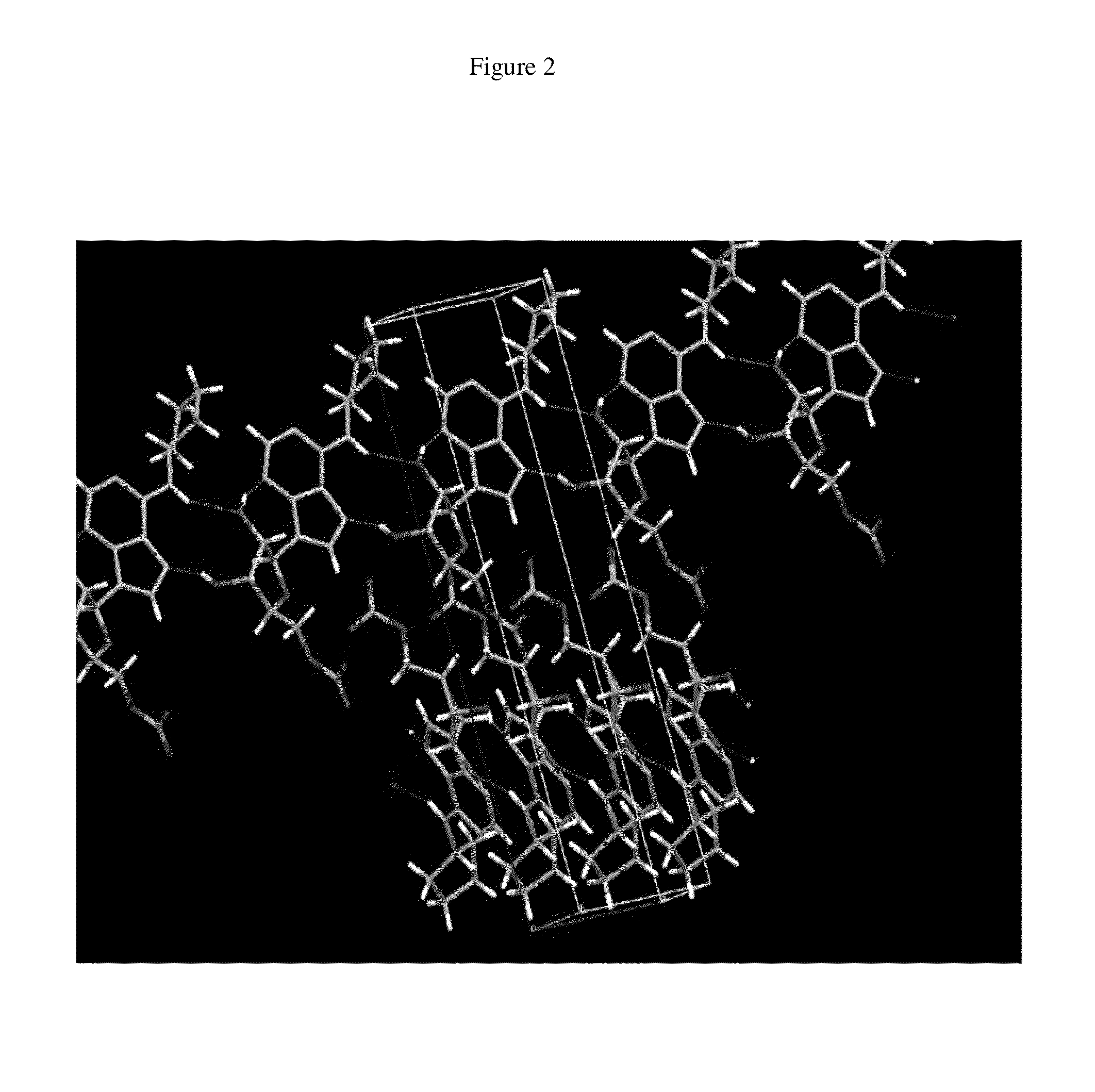
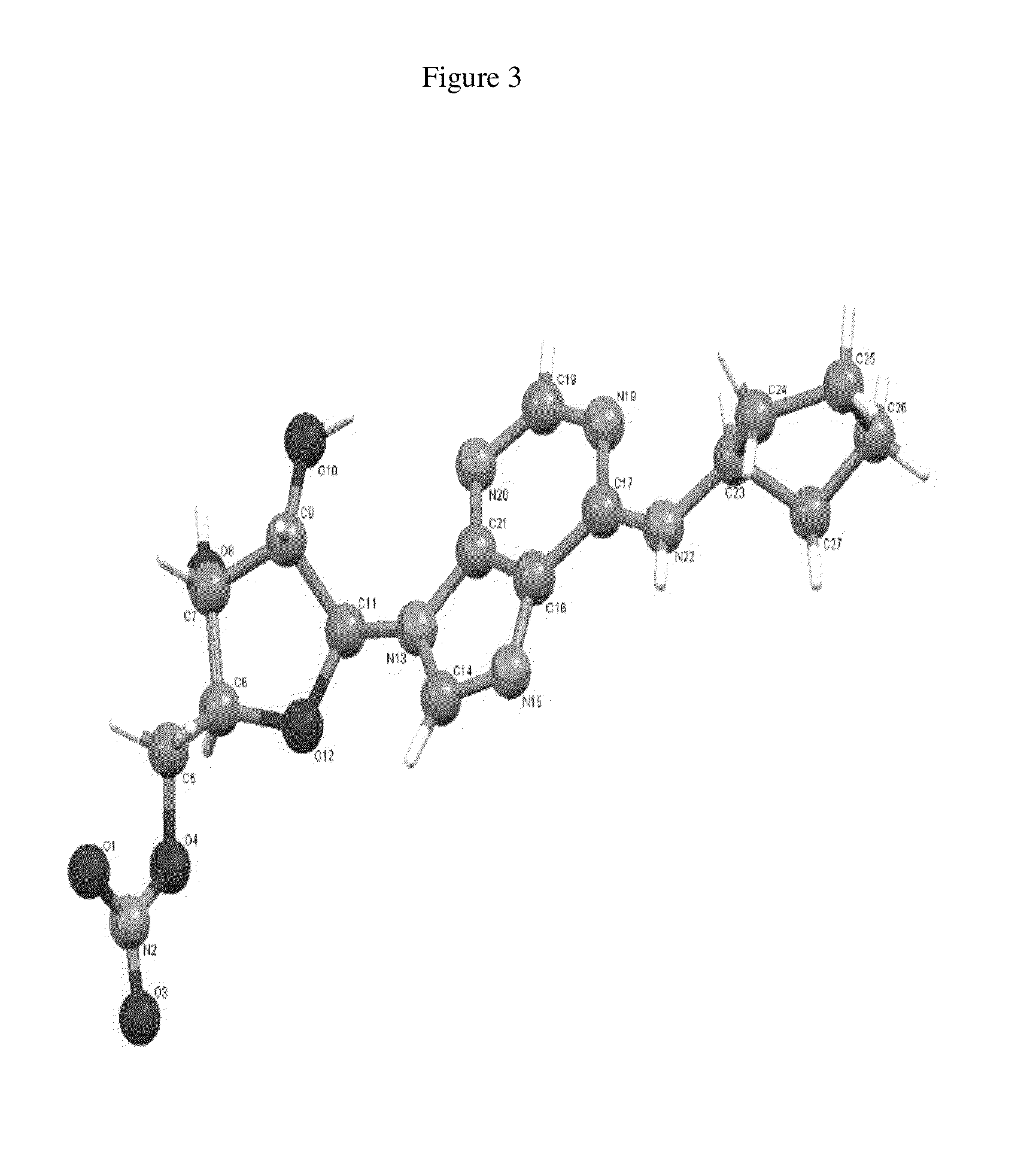
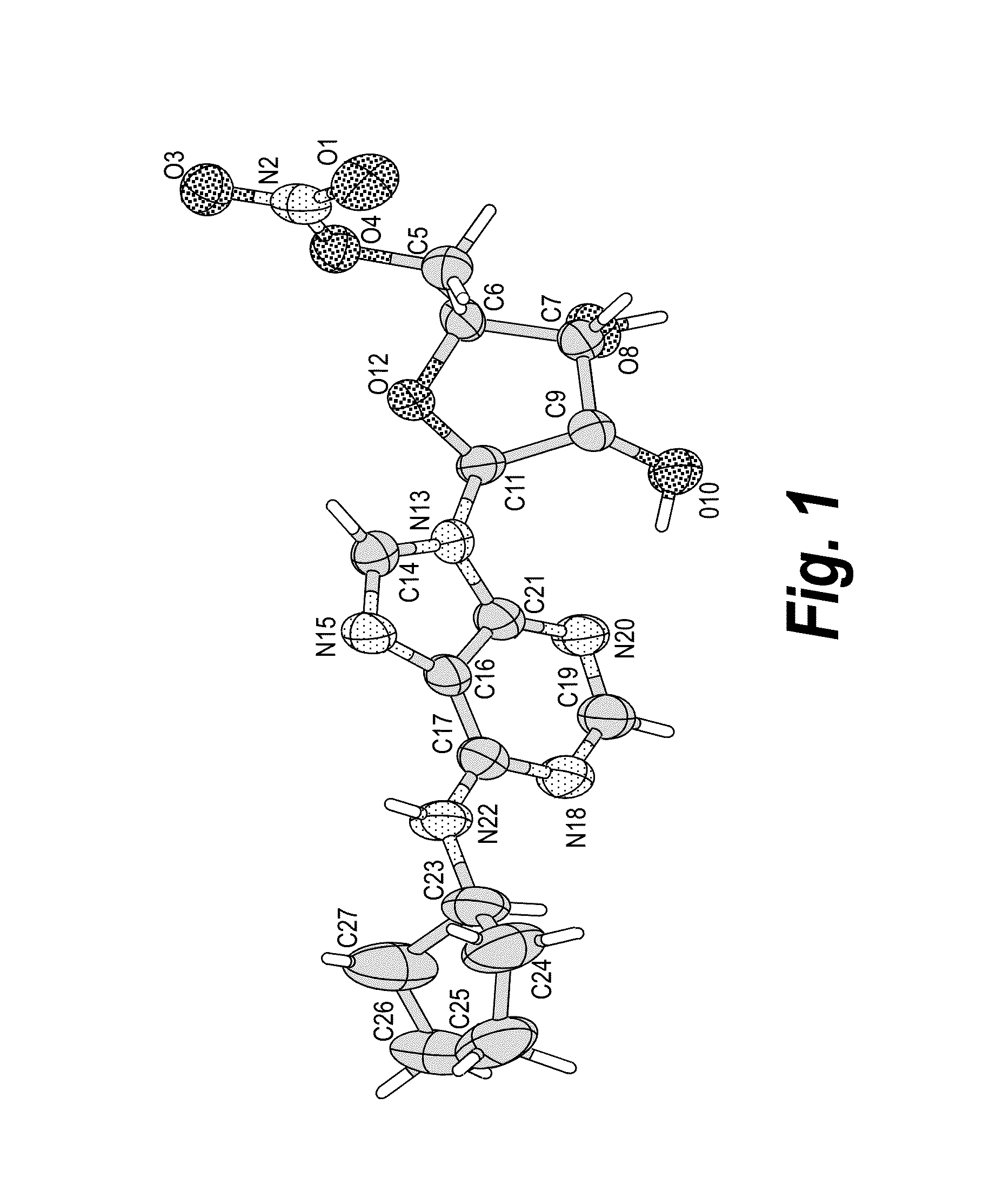
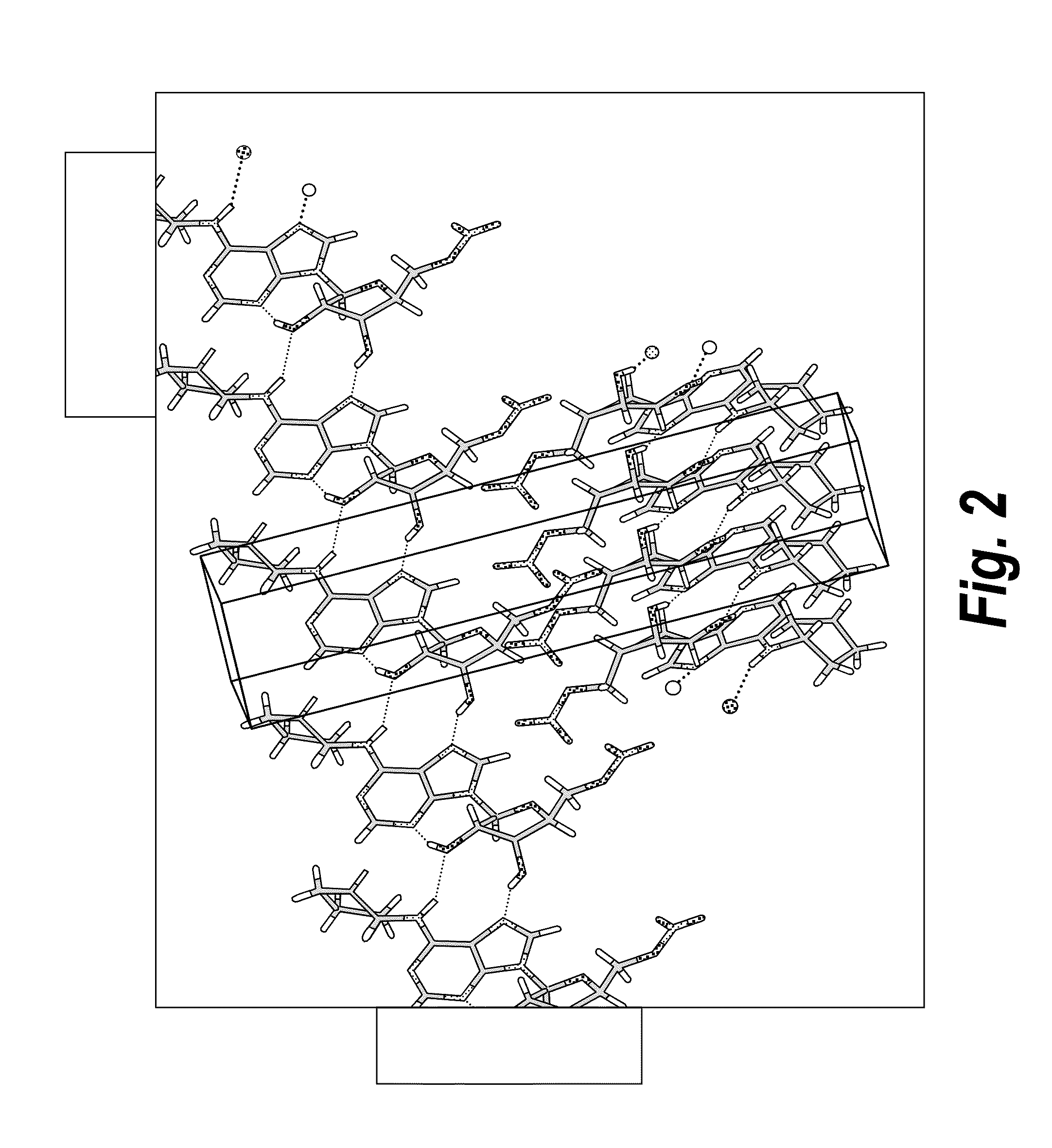
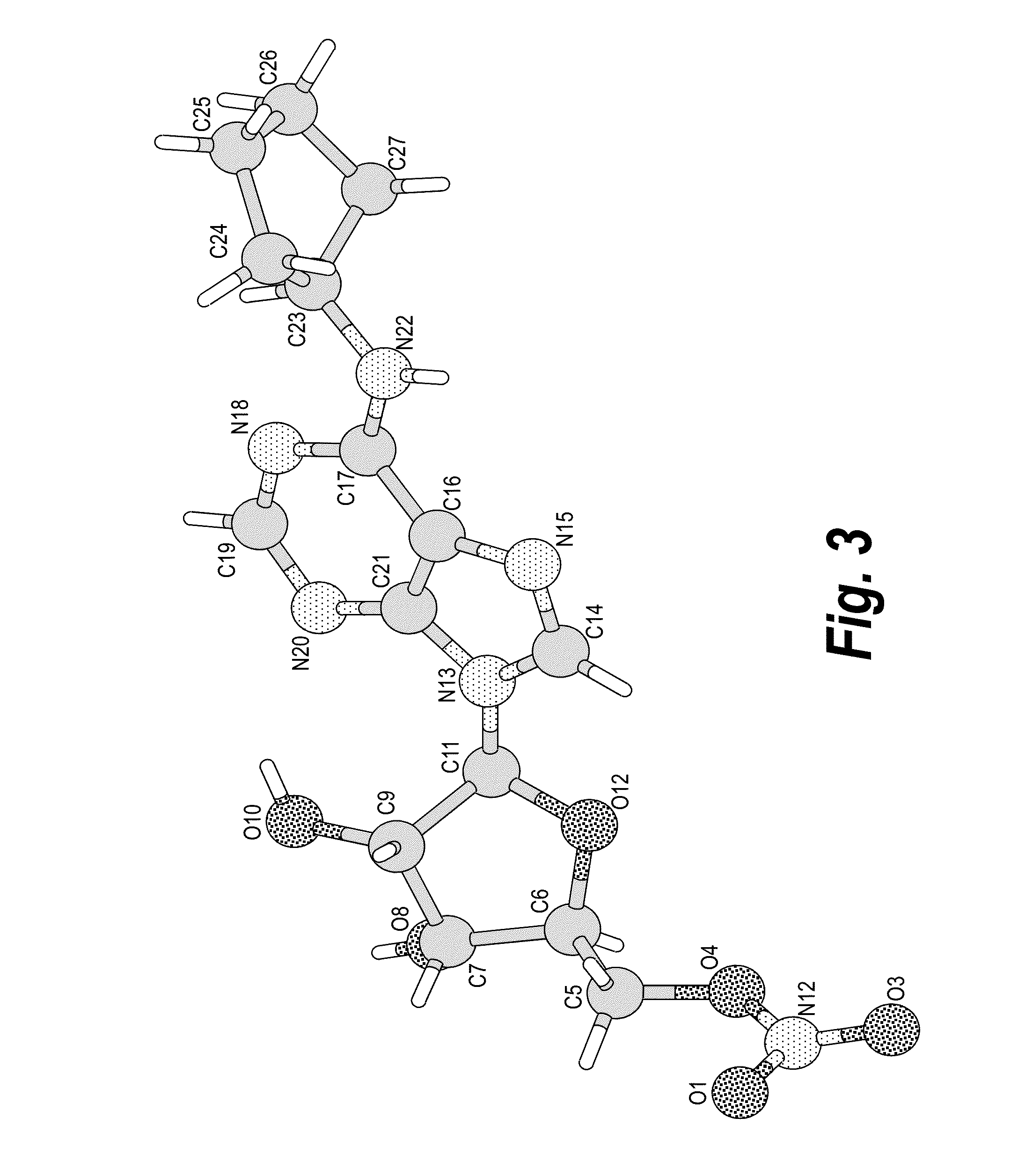
Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)} methyl nitrate and processes of preparation thereof
The present invention provides novel anhydrous polymorph forms of 2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl nitrate (Compound A). The present invention also provides processes for preparation of the anhydrous polymorphic forms of compound A.
Owner:INOTECK PHARMA CORP
Hyperglycemic Sensor Apparatus for Breath Gas Analysis
InactiveUS20180271405A1Diagnostics using spectroscopyDisease diagnosisAcute hyperglycaemiaMonitoring system
A monitoring system is disclosed that includes features for detecting the presence of biomarkers from a gas sample, such as exhaled breath. An assembly includes a plurality of sensors to detect biomarkers present in exhaled breath that are associated with hyperglycemia. The biomarkers include, without limitation, acetone, ethanol, and methyl nitrate.
Owner:CAIRE DIAGNOSTICS INC
Anhydrous polymorphs of [(2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)} methyl nitrate and processes of preparation thereof
The present invention provides novel anhydrous polymorph forms of 2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl) methyl nitrate (Compound A), a selective adenosine A1 receptor agonist with a number of therapeutic uses including the treatment of elevated intra-ocular pressure. Also provided are methods for the preparation of the anhydrous polymorphic forms of compound A, pharmaceutical compositions and methods of treatment.
Owner:INOTECK PHARMA CORP
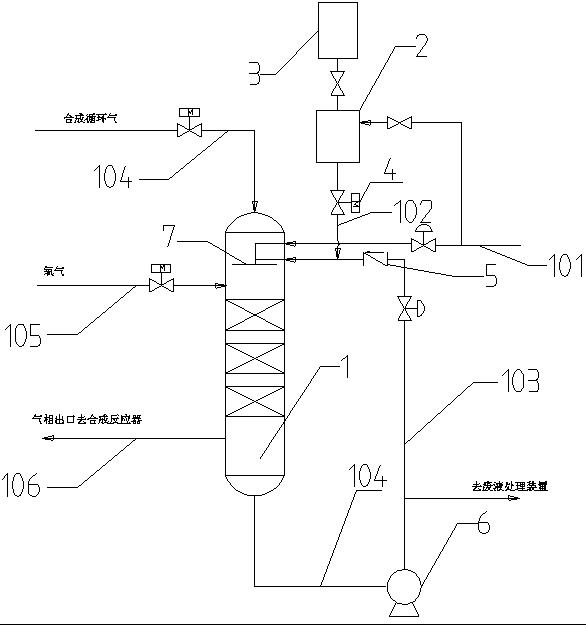
System and method for preventing burning explosion in methyl nitrite preparation process
PendingCN112010756AGuaranteed operational safetyEnsure safetyTransportation and packagingMixer accessoriesNitrationPhysical chemistry
The invention relates to a system and a method for preventing burning explosion in a methyl nitrite preparation process. The system comprises a safety mixing subsystem and a nitrosation reaction tower, the upper part of the nitrosation reaction tower is provided with a methanol inlet (6), the lower part of the nitrosation reaction tower is provided with a reaction feed gas inlet (9), and the safety mixing subsystem is connected with the feed gas inlet (9). Compared with like systems and methods in the prior art, the system and the method of the invention have the advantages of higher safety, capability of ensuring that oxygen and NO recycle gas can be safely, quickly and fully mixed, stable temperature in the nitrosation reaction tower and the like.
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing methyl nitrite by utilizing reaction composite reinforcement
InactiveCN111548274AEfficient preparationCheap manufacturingNitrous acid preparation ester preparationNitrogen oxidesMethyl nitrate
The invention discloses a method for preparing methyl nitrite by utilizing reaction composite reinforcement. The method comprises the following steps: carrying out esterification reaction on oxide, oxygen and methanol, carrying out nitric acid reduction reaction on nitrogen oxide, nitric acid and methyl alcohol, and compositing the esterification reaction and nitric acid reduction reaction to reinforcing the preparation of the methyl nitrite, wherein by-product nitric acid after esterification reaction and excessive methanol after esterification reaction are used as raw materials for nitric acid reduction reaction. According to the invention, the reactions of preparing methyl nitrite by esterification reaction and preparing methyl nitrite by reduction of dilute nitric acid are compositelyreinforced; therefore, an inevitable by-product nitric acid generated by esterification reaction is used as a raw material for dilute nitric acid reduction reaction; in addition, excessive methanol isused as a raw material for subsequent dilute nitric acid reduction, dilute nitric acid and methanol are converted into industrially required methyl nitrite and harmless water, so that the raw material utilization rate of esterification reaction is enhanced, the content of nitric acid in waste liquid after reaction is reduced, and the treatment difficulty and treatment cost of subsequent waste liquid are reduced.
Owner:上海诺哈尔化工技术有限公司
Preparation method and application of methyl nitrite
InactiveCN112142600AEmission reductionReduce pollutionMolecular sieve catalystsOrganic compound preparationPtru catalystMethyl nitrate
The invention discloses a preparation method and application of methyl nitrite, wherein the preparation method at least comprises the step: sequentially contacting a raw material containing nitric acid and methanol with a reduction reaction catalyst and an esterification reaction catalyst in a reaction zone to obtain the methyl nitrite. The method not only can improve the economic benefit and fully utilize the waste liquid, but also can reduce the pollution to water resources. The invention also discloses the application of the methyl nitrite obtained by the method in circularly entering coal-to-ethylene glycol.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
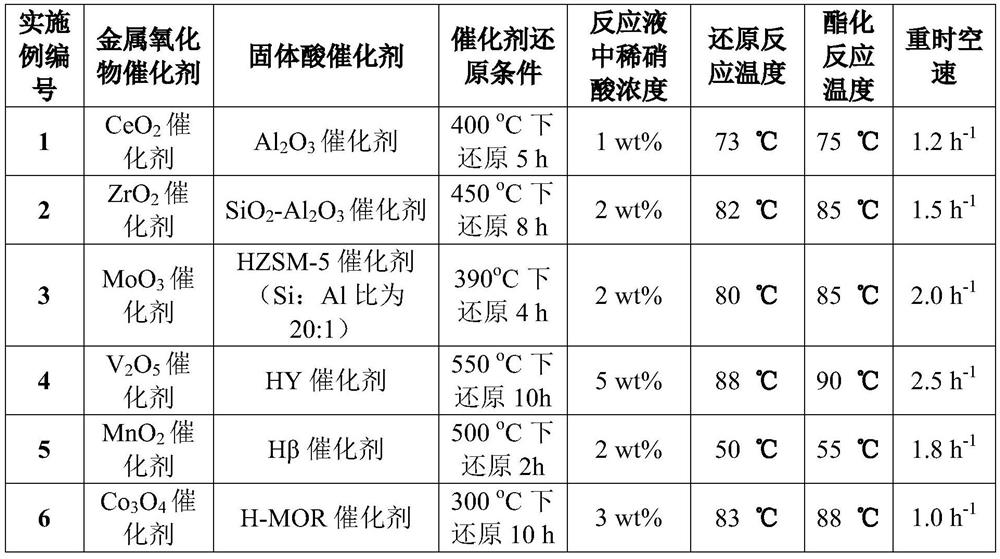
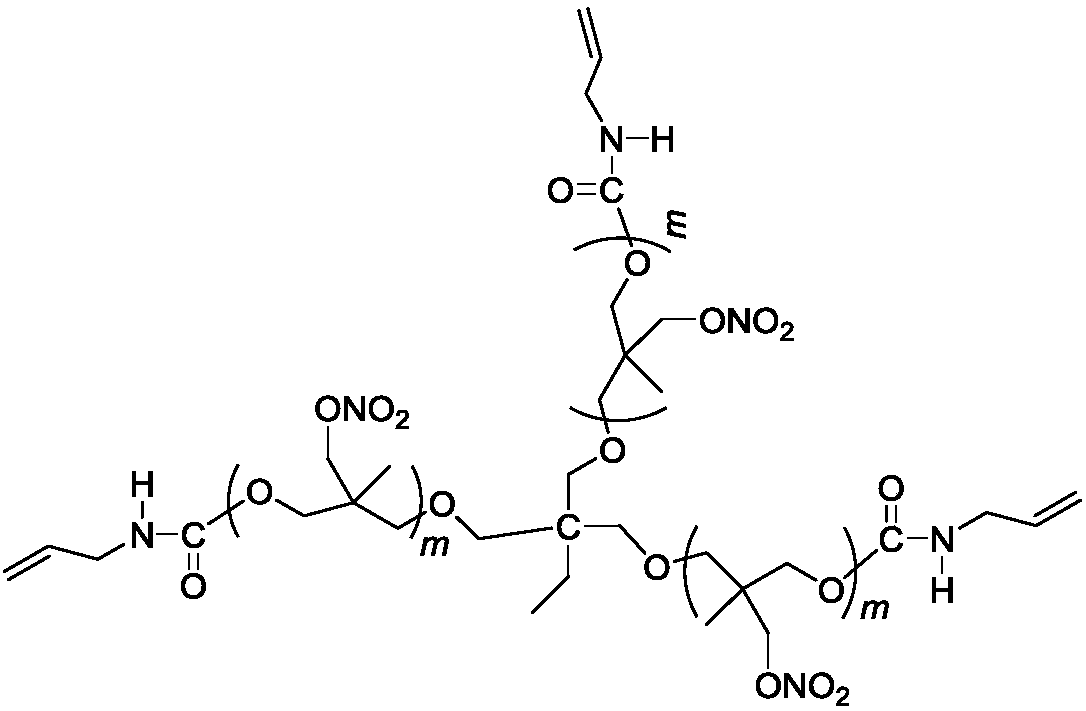
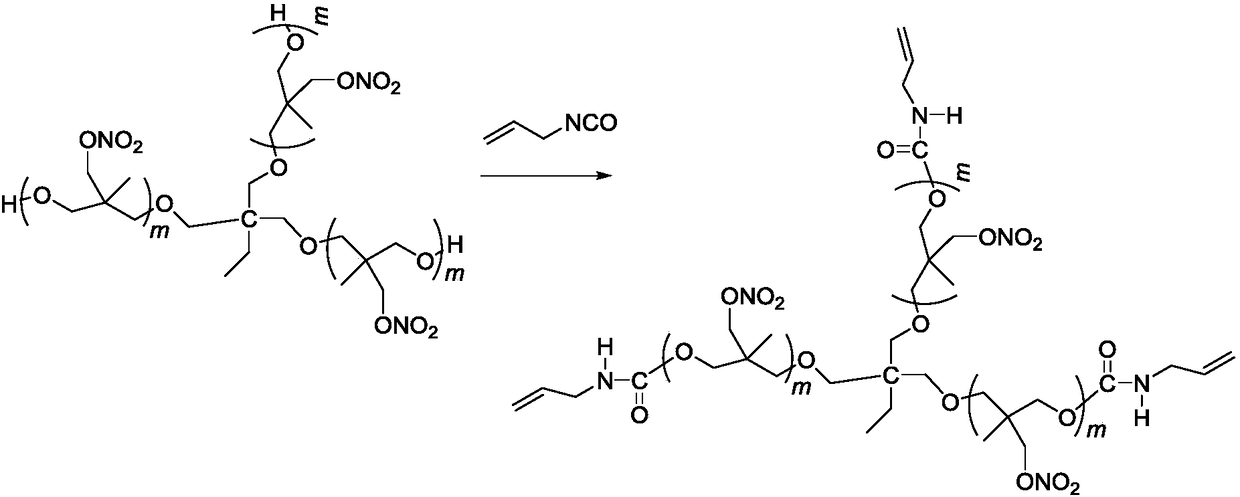
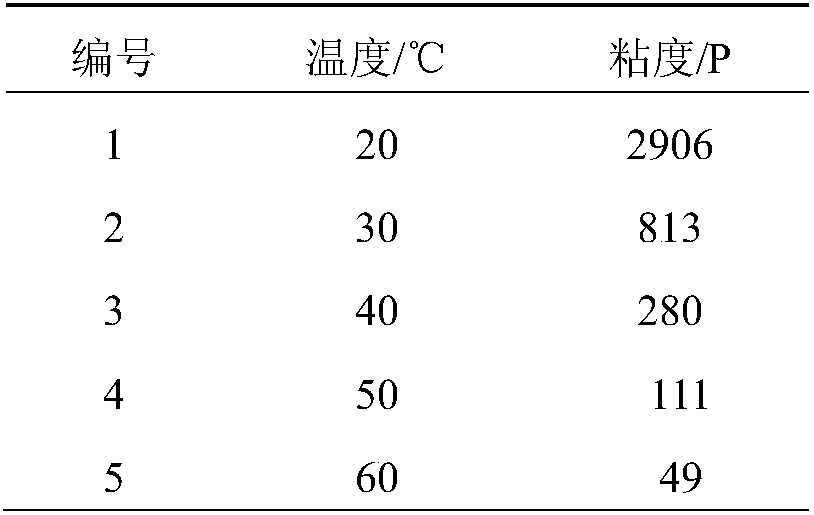
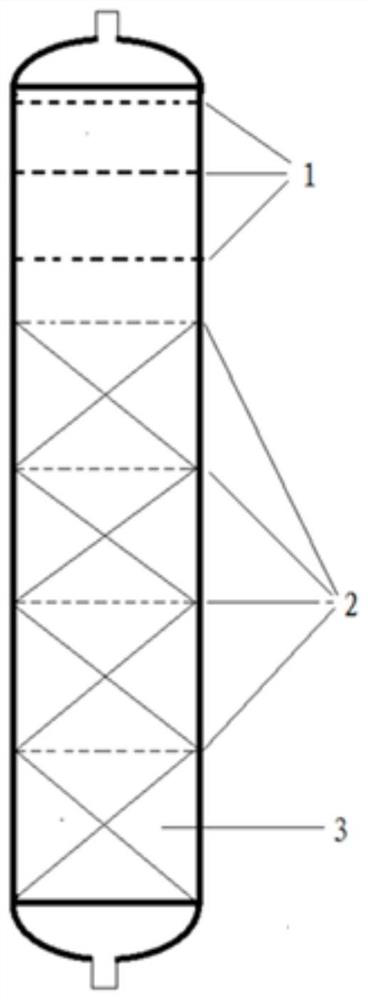
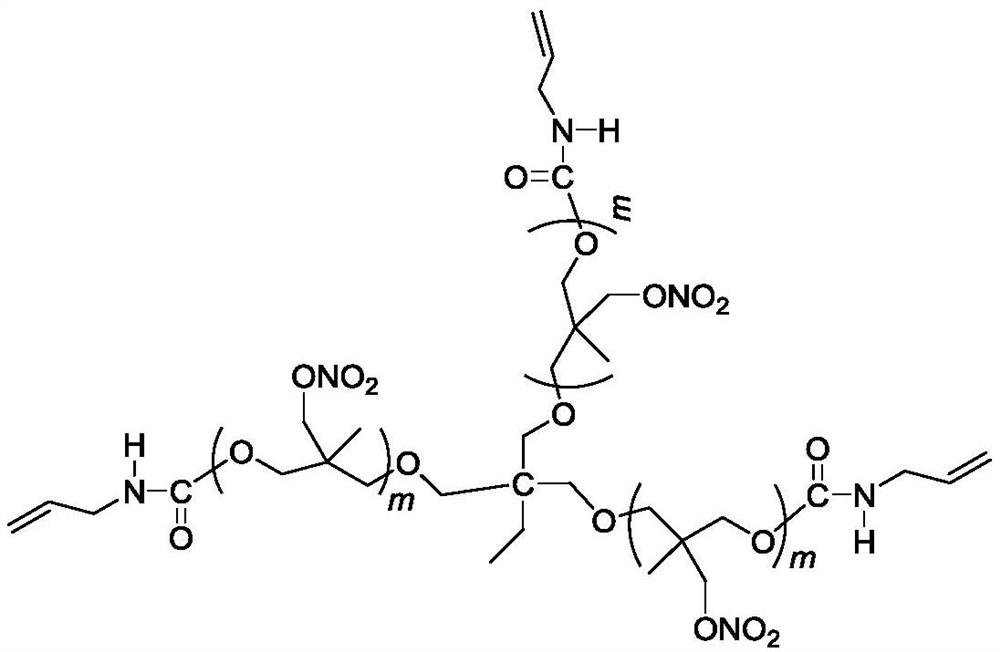
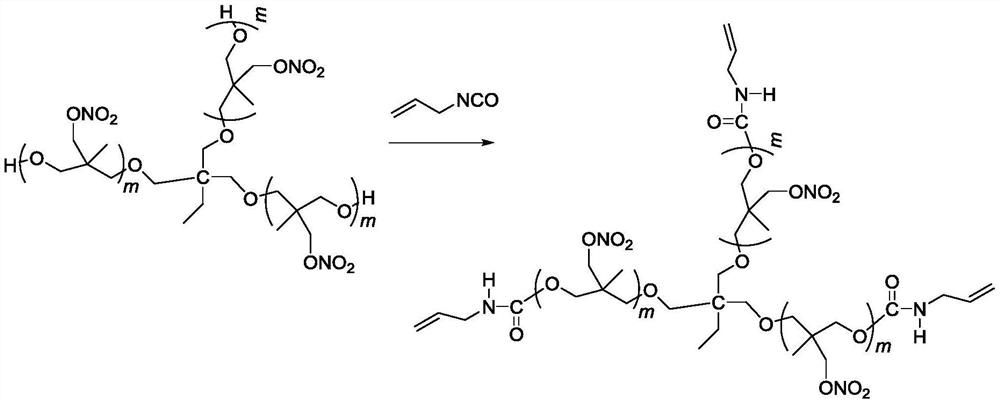
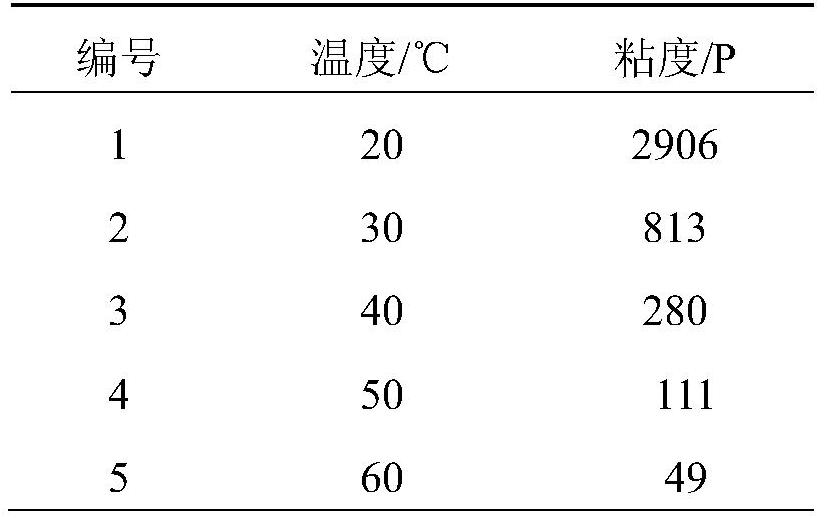
Three-degree-of-functionality terminal alkenyl energetic adhesive and synthesis method thereof
The invention discloses a three-degree-of-functionality terminal alkenyl energetic adhesive and a synthesis method thereof. The structural formula of the three-degree-of-functionality terminal alkenylenergetic adhesive is shown in a formula (I). The synthesis process comprises the following steps: taking three-degree-of-functionality poly3-methyl nitrate-3-methoxy heteroxane as a raw material, adding 3-propylene isocyanate and performing addition reaction to obtain the three-degree-of-functionality terminal alkenyl energetic adhesive. The synthesis method is convenient to prepare on a large scale, and double bonds give room-temperature curing ability to the adhesive. The three-degree-of-functionality terminal alkenyl energetic adhesive is mainly applied to a composite solid propellant. The formula is as shown in the description, wherein m is an integer of 5 to 10.
Owner:XIAN MODERN CHEM RES INST
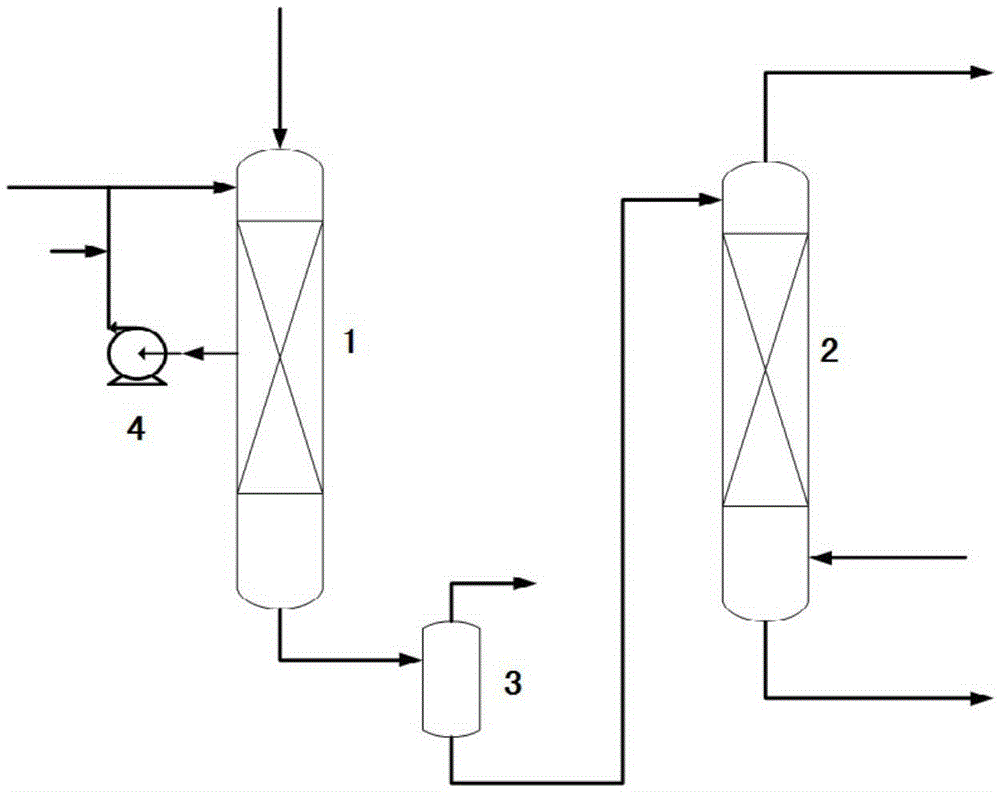
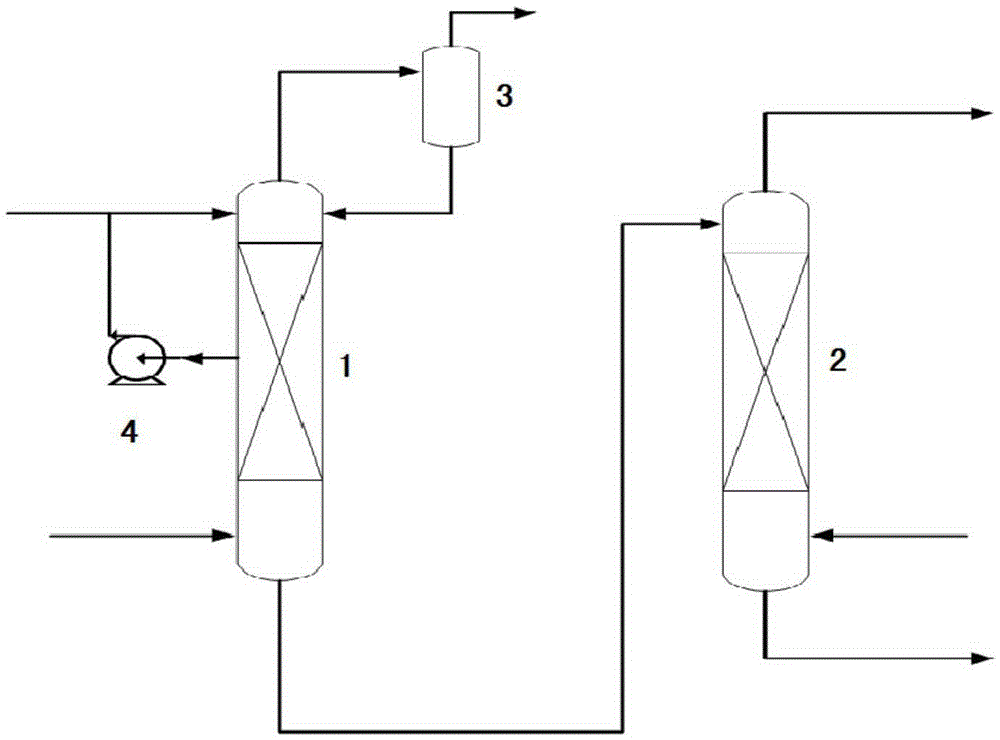
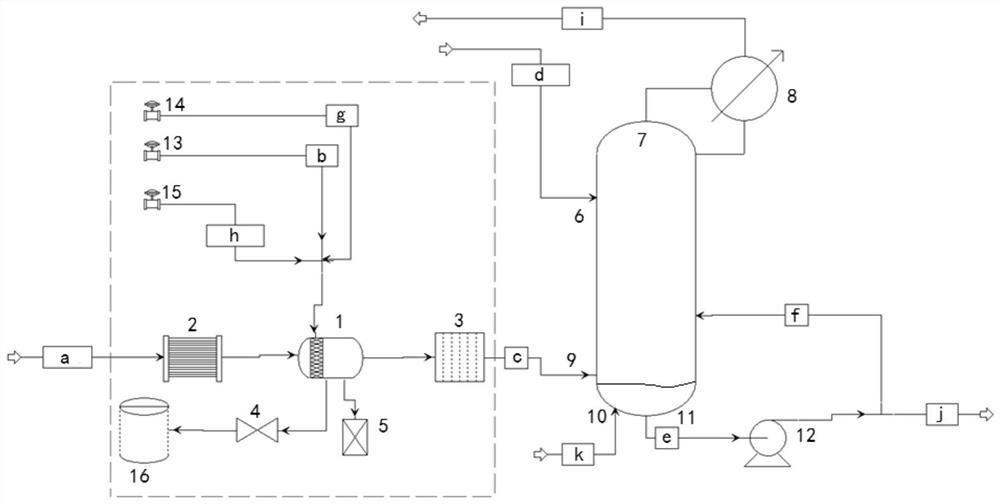
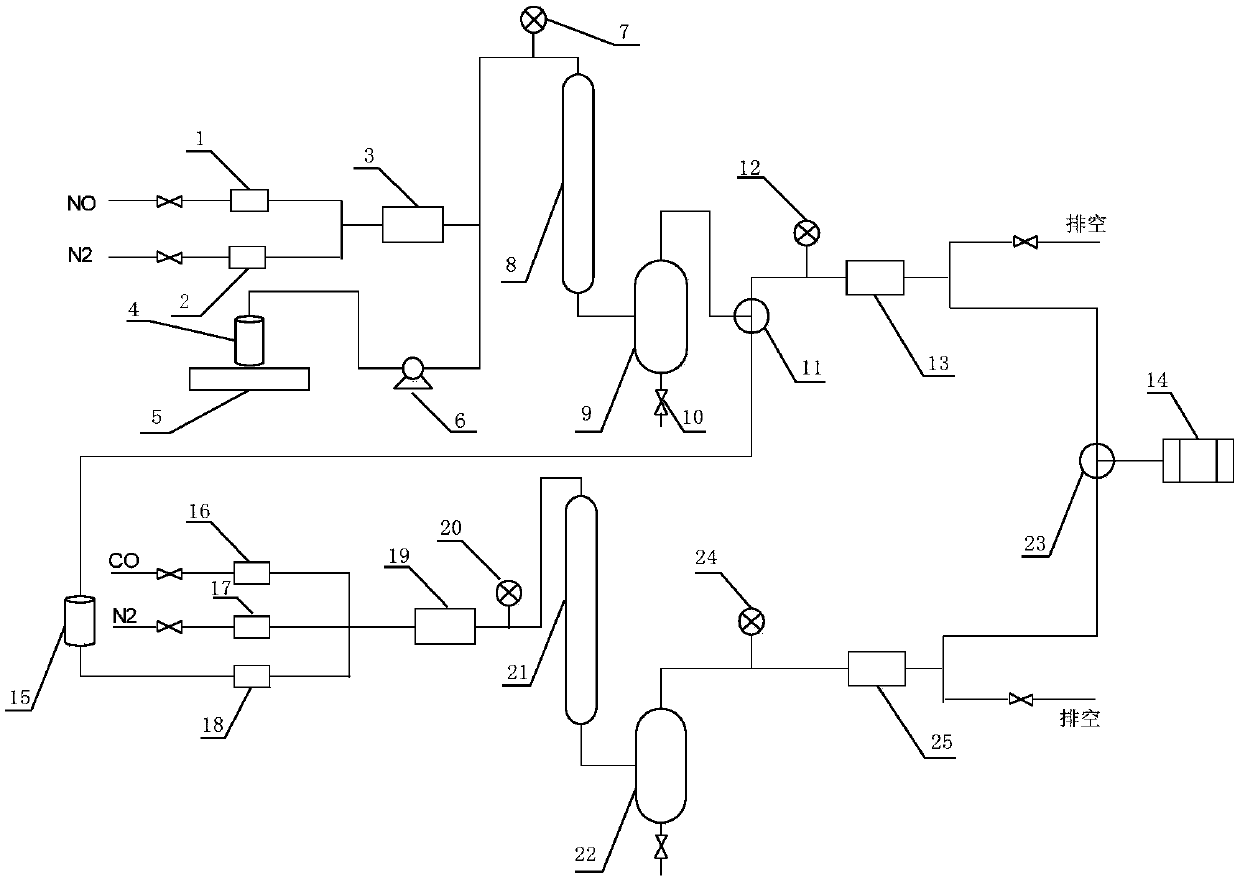
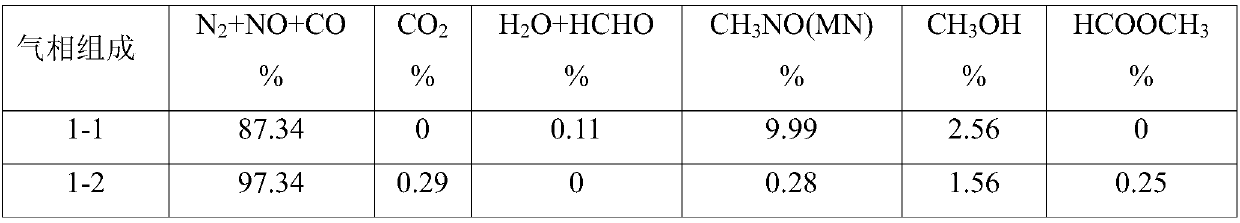
Device for continuously producing methyl nitrite gas and application of device
PendingCN110818568AAchieve continuous supplyChemical analysis using catalysisNitrous acid preparation ester preparationGas analysisMethyl nitrate
The invention provides a device for continuously producing methyl nitrite gas and an application of the device. The device comprises a methyl nitrite gas preparation system, a gas analysis system anda first fluid delivery control unit; the methyl nitrite gas preparation system is used for continuously producing methyl nitrite gas; the gas analysis system is used for obtaining the concentration ofthe methyl nitrite gas, wherein the methyl nitrite gas is from the methyl nitrite gas preparation system; and the first fluid delivery control unit is connected with the methyl nitrite gas preparation system and the gas analysis system, is used for making communication between the methyl nitrite gas preparation system and the gas analysis system to provide a to-be-analyzed gas sample for the gasanalysis system, and is also used for controlling product output of the methyl nitrite gas preparation system. According to the invention, stable supply of methyl nitrite gas is realized, the methyl nitrite gas preparation system and a catalytic reaction evaluation system taking methyl nitrite as a raw material are further connected in series, and stability and service life evaluation of a nitricacid reduction reaction for preparing methyl nitrite gas and supply of a stable gas source for a catalytic reaction are realized.
Owner:SHANGHAI HUAYI ENERGY CHEM
Diesel oil efficiency-increase and emission-reduction additive
InactiveCN106244265APrevent Storage StratificationImprove low temperature performanceLiquid carbonaceous fuelsFuel additivesParticulatesExhaust gas emissions
The invention relates to the technical field of fuel additives, and in particular, relates to a diesel oil efficiency-increase and emission-reduction additive prepared from the following raw materials in parts by weight: 8-24 parts of 1-hexyl-3-methylimidazolium bromide, 5-21 parts of dimethylformamide, 2-10 parts of methyl benzoate, 1-5 parts of succimide, 1-7 parts of methyl silicone oil, 2-10 parts of 2-diethylaminopyridine, 1-5 parts of 3-methylphenol, 4-14 parts of methyl nitrate, and 1-9 parts of ethyl nitrate. The diesel oil efficiency-increase and emission-reduction additive has the advantages of good cleaning purification and anti-corrosion effects, effectively saves diesel oil, reduces exhaust gas emission, and significantly reduces emissions of black smoke and particulate matters.
Owner:GUANGXI DONGQI ENERGY TECH CO LTD
Preparation and application of dimethyl carbonate catalyst
PendingCN112246240AHigh selectivityImprove performanceOrganic compound preparationCatalyst activation/preparationCarbonyl groupMethyl nitrate
The invention relates to preparation and application of a dimethyl carbonate catalyst, and belongs to the technical field of catalyst preparation. According to the present invention, the alumina carrier is modified, the highly-dispersed nanometer palladium loaded on the surface of the carrier is prepared through the room temperature impregnation method so as to obtain the catalyst with excellent performance, the catalyst is used for carbonylation synthesis of dimethyl carbonate from carbon monoxide and methyl nitrite; the conversion rate of methyl nitrite achieves more than or equal to 60% andthe selectivity of dimethyl carbonate is higher than 80%, so that the alumina-based palladium catalyst prepared by the method is a catalyst with excellent conversion rate and selectivity.
Owner:JIANGSU JINJU ALLOY MATERIAL
Method for preparing methoxyamine, method for preparing methoxyamine hydrochloride
ActiveCN112125822BImprove conversion efficiencyEasy to operateOrganic chemistryPtru catalystMethyl nitrate
The present application discloses a method for preparing methoxyamine, which at least includes: contacting a raw material gas containing methyl nitrite and a reducing agent with a reduction reaction catalyst in a reactor to perform a reduction reaction to obtain methoxyamine. The method can fully utilize the important intermediate methyl nitrite in the coal-to-ethylene glycol process, and the conversion rate of the methyl nitrite is high. The present application also provides a preparation method of methoxyamine hydrochloride obtained by the above method as raw material.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Safety device for regeneration tower for coal-to-ethylene glycol methyl nitrite esterification
ActiveCN107056619BMove out quicklySmooth production processNitrous acid preparation ester preparationGas phaseMethyl nitrate
The invention relates to a methyl nitrite esterification regenerating column safety device for producing glycol from coal, which comprises an esterification regenerating column, a methanol storage tank and an accumulator, wherein the methanol storage tank is arranged at the column top of the esterification regenerating column and is connected with the esterification regenerating column via a connecting pipeline, and the esterification regenerating column is connected with a synthesis recycle gas inlet pipeline, an oxygen inlet pipeline and a gas phase outlet pipeline; the inlet of the methanol storage tank is connected with a methanol inlet pipeline and the outlet thereof is connected with a methanol outlet pipeline, the methanol outlet pipeline is connected to a first stage circulating methanol main pipe and enters the esterification regenerating column via a methanol distributor for spraying, and the methanol inlet pipeline is connected with the methanol distributor via a pipeline; and the accumulator is arranged at the upper end of the methanol storage tank, and the methanol storage tank and the accumulator are connected with valves via pipelines. The invention aims to provide a methyl nitrite esterification regenerating column safety device for producing glycol from coal, which can effectively solve the problem of unusual service conditions due to power outage, steam supply stop and water supply stop, and ensure safe and efficient emergency disposal of the system.
Owner:安阳永金化工有限公司
Method of producing toltrazuril
Owner:PU LIKE BIO ENG +1
A kind of method utilizing waste and old palladium catalyst to synthesize dimethyl oxalate catalyst
ActiveCN108452810BHigh activityImprove adhesionCatalyst activation/preparationPreparation by carbon monoxide or formate reactionPtru catalystPalladium catalyst
The invention discloses a method for synthesizing a dimethyl oxalate catalyst by using a waste palladium catalyst. The leaching method is used to recover the metal palladium in the waste palladium-alumina catalyst. The remaining alumina pellets are washed, dried and roasted, and then reused. Load palladium and additives to prepare a catalyst for the reaction between methyl nitrite and CO to generate dimethyl oxalate. The extracted alumina balls are reused as catalyst carriers. The catalyst activity is good, the conversion rate of methyl nitrite and the selectivity of the product dimethyl oxalate are very good, the service life is long, and the adhesion of the carrier beads to palladium is significantly enhanced. The loss of precious metal palladium in the catalyst palladium-alumina is greatly suppressed.
Owner:河南能源集团研究总院有限公司
Catalyst for CO gas-phase coupling synthesis of dimethyl carbonate, preparation method and application of the catalyst
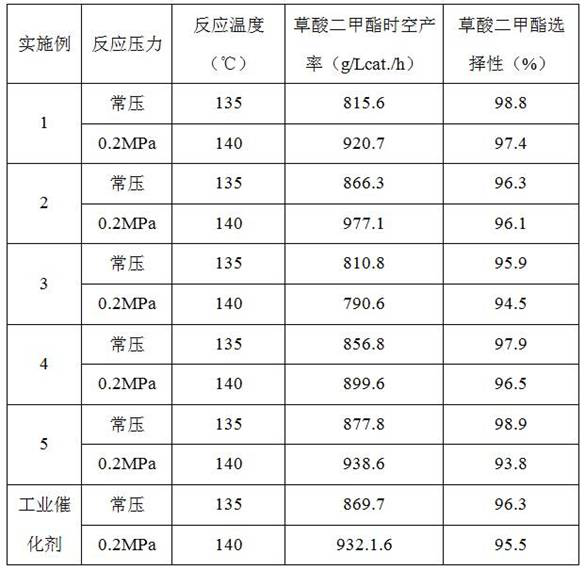
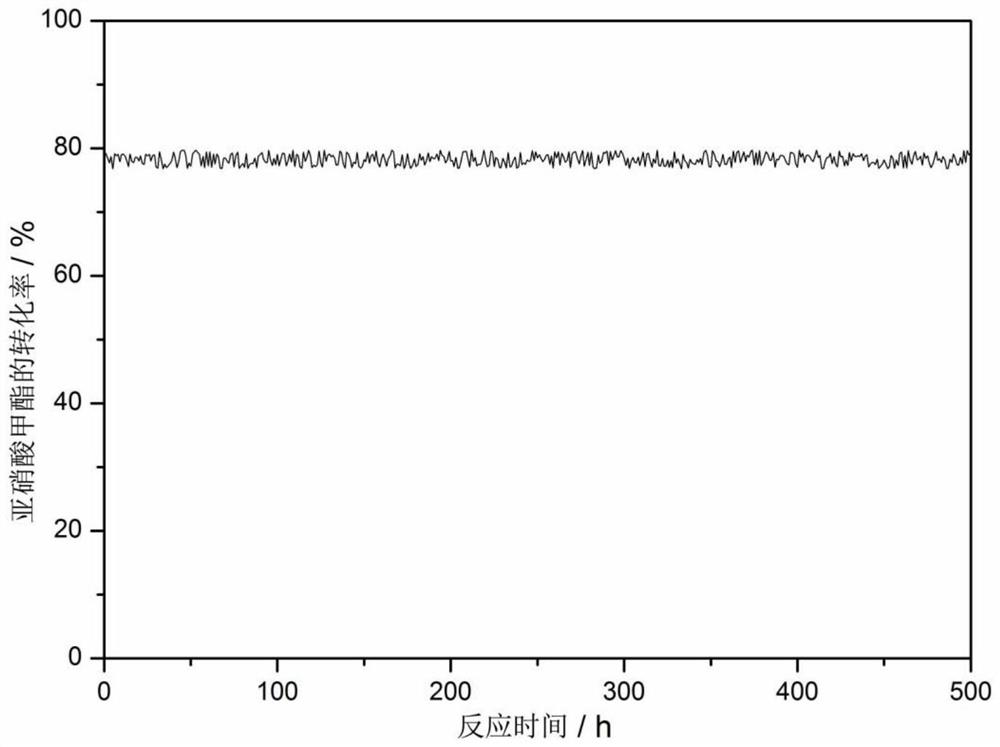
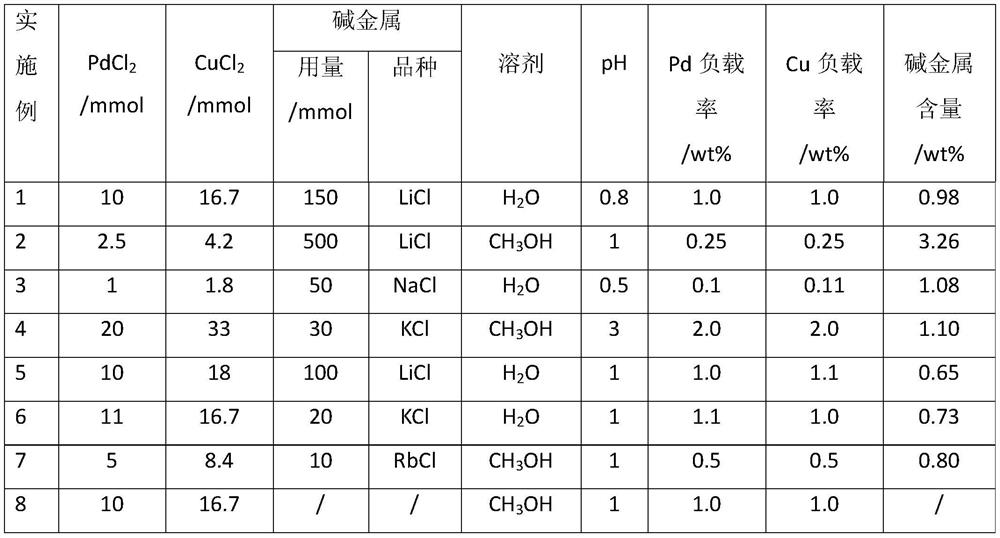
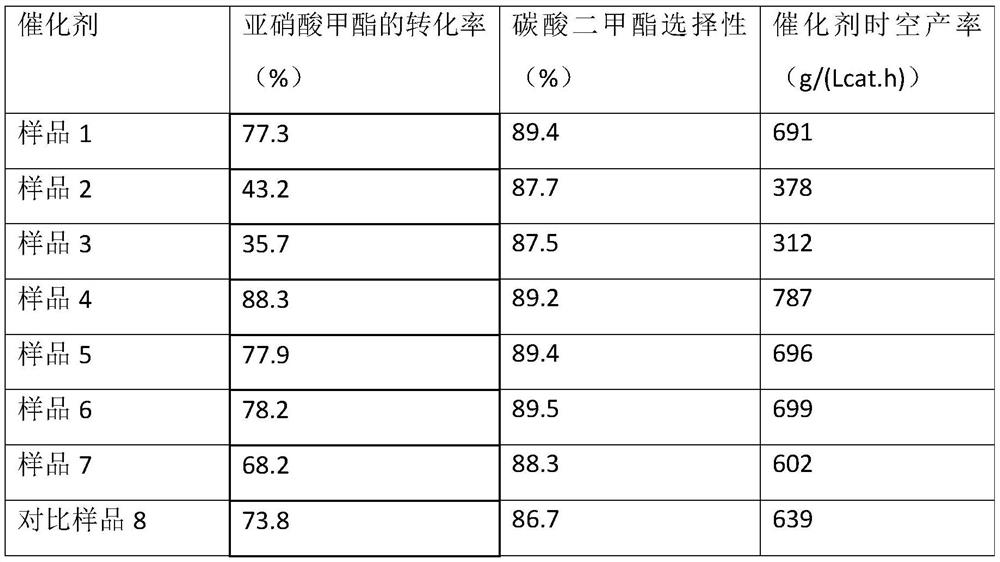
ActiveCN107376954BEasy to useSolution to short lifePhysical/chemical process catalystsOrganic compound preparationPtru catalystNitrite
The invention discloses a catalyst for gas phase synthesis of dimethyl carbonate by CO and methyl nitrite under low temperature and low pressure conditions, a preparation method of the catalyst and an application thereof. The catalyst is γ-Al 2 o 3 A wacker-type catalyst that supports Pd‑Cl‑Cu and is doped with alkali metal elements; the γ‑Al of the catalyst 2 o 3 The specific surface area of the carrier is 20‑200m 2 / g, the pore volume is 0.1‑1.5m 2 / g, the pore size is 3-20nm; the alkali metal element is one or more of Li, Na, K, Rb, and the alkali metal element content is 0.1wt%-5.0wt%. The catalyst of the present invention can be applied to CO and methyl nitrite gas-phase synthesis of dimethyl carbonate under low temperature and low pressure conditions. When it is used at low pressure and low temperature, it has high selectivity and space-time yield, and is suitable for industrialized CO and nitrite. Dimethyl carbonate is synthesized from methyl nitrate in low-temperature and low-pressure gas phase; and it has a longer service life than common noble metal catalysts.
Owner:NINGBO JINYUANDONG PETROCHEM ENG TECH
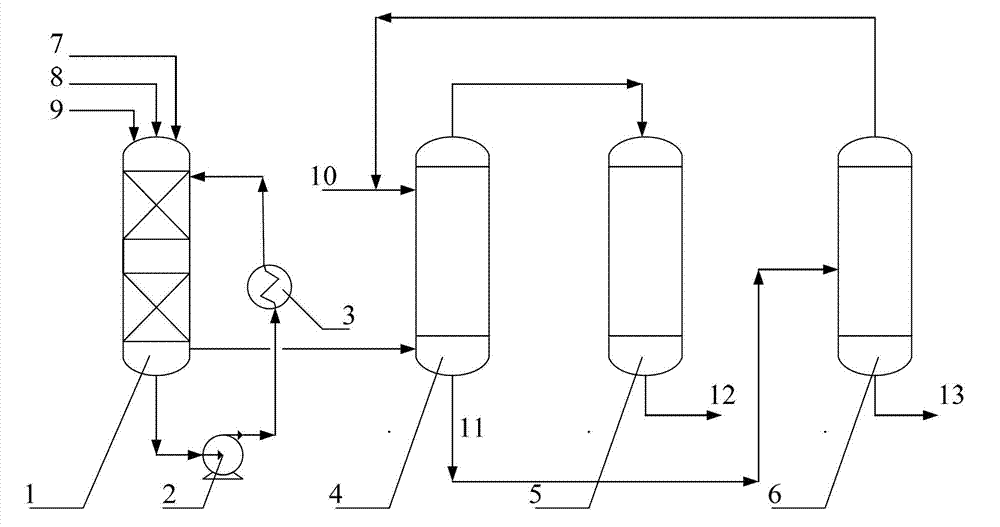
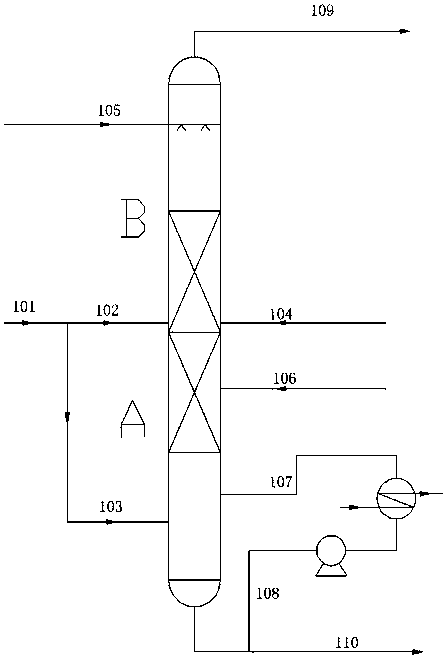
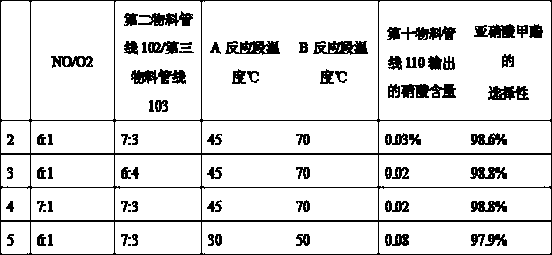
Method and device for safely preparing methyl nitrite in oxidative esterification reactor and application thereof
PendingCN113912498ARealize safe industrial productionAvoid explosionOrganic compound preparationHydroxy compound preparationPhysical chemistryMethyl nitrate
The invention relates to the field of ethylene glycol synthesis, and discloses a method for safely preparing methyl nitrite in an oxidative esterification reactor, which comprises the following steps: introducing fresh methanol, nitric oxide and oxygen into the oxidative esterification reactor, carrying out oxidative esterification reaction at a set temperature T and a set pressure P, recycling cold methanol from the unreacted gas phase, and returning the recycled cold methanol to the oxidative esterification reactor to participate the oxidative esterification reaction. The method comprises measuring the temperature and / or pressure in the oxidative esterification reactor regularly or irregularly, and controlling the temperature and / or pressure in the oxidative esterification reactor in a hierarchical control mode, thus maintaining the temperature and the pressure in a safe range. The method and the oxidative esterification reactor provided by the invention can effectively prevent burning explosion in the preparation process of the methyl nitrite, so that the use of the methyl nitrite in a coupling section is ensured, and the safe industrial production of preparing the ethylene glycol from the synthesis gas is realized.
Owner:CHINA PETROLEUM & CHEM CORP +1
A kind of trifunctional end alkenyl energetic adhesive and its synthesis method
The invention discloses a three-degree-of-functionality terminal alkenyl energetic adhesive and a synthesis method thereof. The structural formula of the three-degree-of-functionality terminal alkenylenergetic adhesive is shown in a formula (I). The synthesis process comprises the following steps: taking three-degree-of-functionality poly3-methyl nitrate-3-methoxy heteroxane as a raw material, adding 3-propylene isocyanate and performing addition reaction to obtain the three-degree-of-functionality terminal alkenyl energetic adhesive. The synthesis method is convenient to prepare on a large scale, and double bonds give room-temperature curing ability to the adhesive. The three-degree-of-functionality terminal alkenyl energetic adhesive is mainly applied to a composite solid propellant. The formula is as shown in the description, wherein m is an integer of 5 to 10.
Owner:XIAN MODERN CHEM RES INST
Y molecular sieve and preparation method for carbonylation of methyl nitrite to synthesize dimethyl carbonate
ActiveCN110844918BImprove conversion rateHigh space-time yieldCatalyst carriersMolecular sieve catalystsPtru catalystMethyl nitrate
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
A kind of method for preparing methyl nitrite from nitric acid
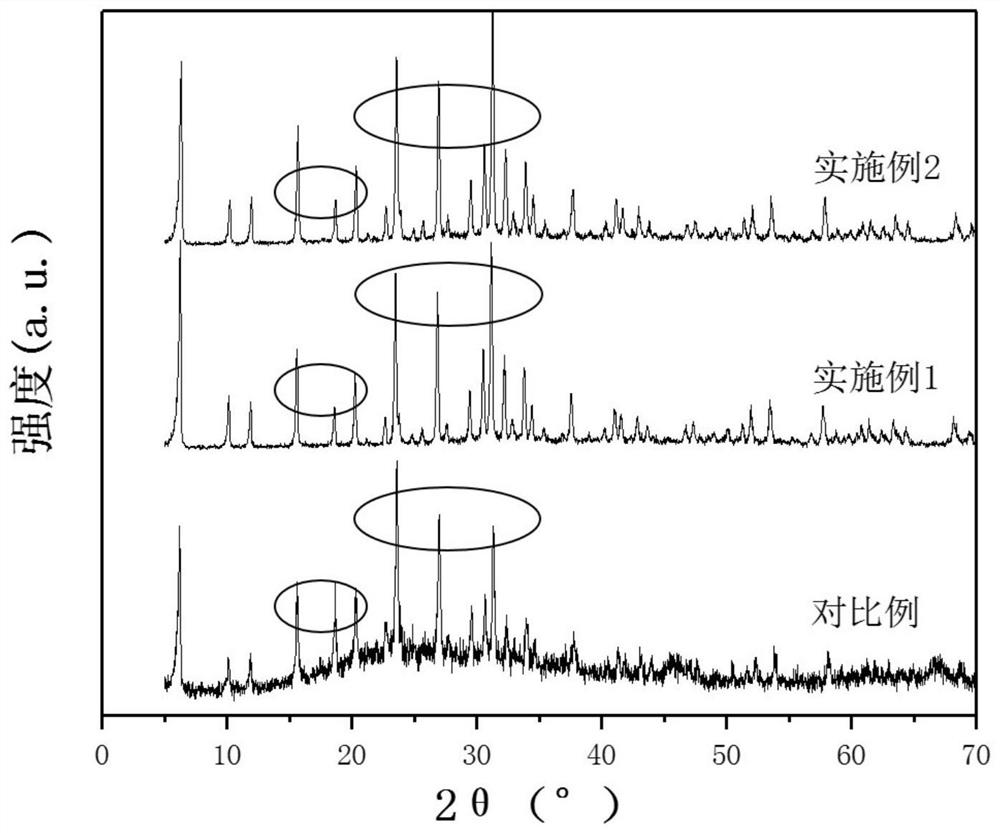
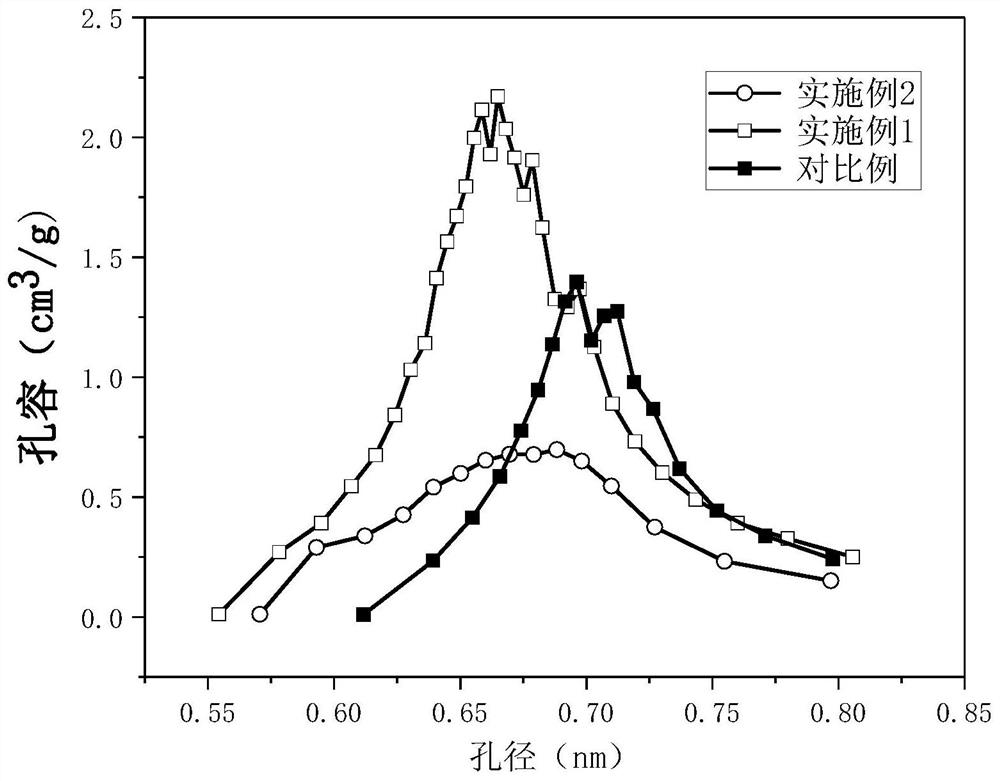
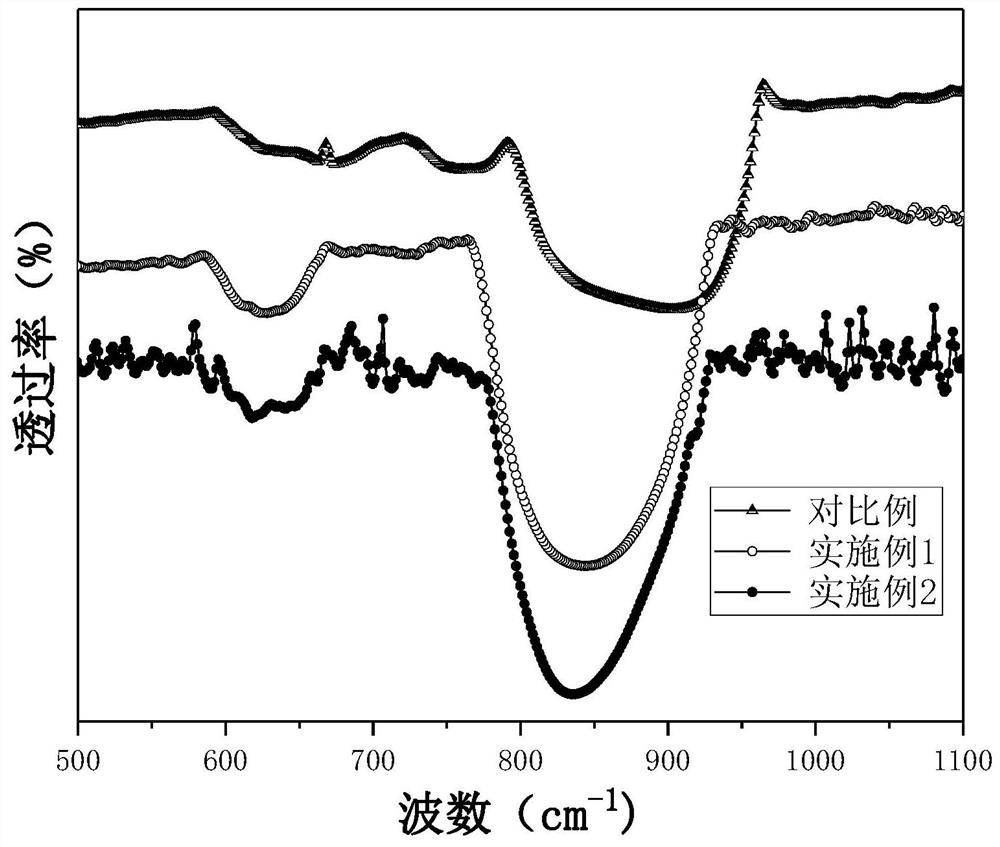
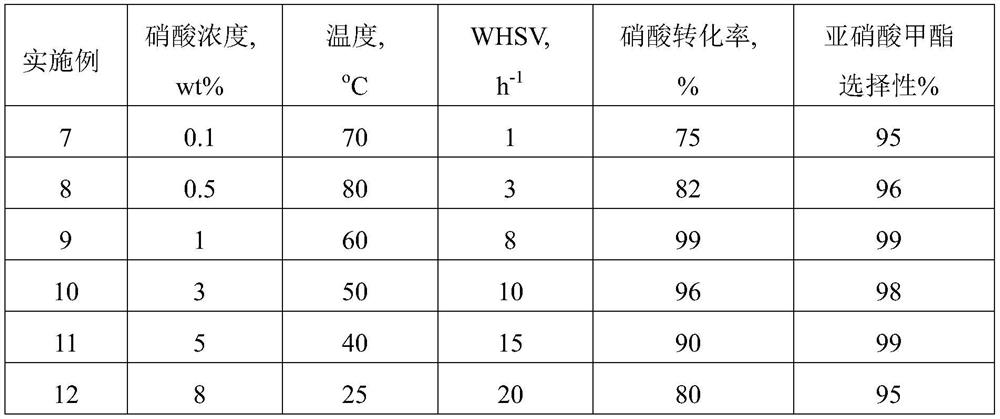
ActiveCN111943851BHigh strengthImprove stabilityPhysical/chemical process catalystsNitrous acid preparation ester preparationCarbide siliconPtru catalyst
The present application discloses a method for preparing methyl nitrite with dilute nitric acid. The method comprises: in a fixed bed reactor, contacting raw materials containing nitric acid and methanol with a catalyst to react to obtain methyl nitrite. Wherein, the catalyst has a core-shell structure; the inner core of the catalyst includes a silicon-containing inorganic compound; the silicon-containing inorganic compound includes at least one of silicon dioxide and silicon carbide; the outer shell of the catalyst includes nitrogen-doped carbon ; Metal elements are loaded on the nitrogen-doped carbon; the metal elements are selected from at least one of ruthenium and gold. When dilute nitric acid and methanol are reacted to prepare methyl nitrite, using the catalyst, the conversion rate of nitric acid and the selectivity of methyl nitrite can reach over 99% at the highest.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of method that microreactor synthesizes methyl nitrite
ActiveCN107793316BImprove mixing efficiencyEfficient responseChemical/physical/physico-chemical microreactorsNitrous acid preparation ester preparationMicroreactorNitration
The invention discloses a method for synthesizing methyl nitrite through a microreactor. The method is mainly characterized by comprising the following steps: (a) firstly pre-reacting NO and O2 in a shell and tube reactor to generate a mixed gas containing oxynitride; and (b) carrying out nitratlon reaction via the mixed gas and methyl alcohol in a micro-channel reactor which is a single-channel reactor or a multi-channel reactor, and generating the methyl nitrite, wherein the hydraulic diameter of each micro reaction channel is in a range of 100-3000 microns, the gas-phase space velocity in the microreactor is 2000-50000h<-1>, the content of N2 in a gas phase is 40-70%; the volume ratio of the NO to the O2 is 4-7; the mole ratio of the methyl alcohol to the feedstock NO is 1-2; the yieldof the product methyl nitrite based on the O2 is greater than 70% after reaction. According to the reaction process, the material residence time is short; the side reaction is effectively inhibited; the generation of HNO3 and the like is reduced; the corrosion to the equipment is low; meanwhile, the characteristics of efficient mass transfer and heat transfer of the micro-channel reactor are used;the process has high operability and safety.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)] methyl nitrate and processes of preparation thereof
The present invention provides novel anhydrous polymorph forms of 2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl nitrate (Compound A). The present invention also provides processes for preparation of the anhydrous polymorphic forms of compound A.
Owner:INOTECK PHARMA CORP
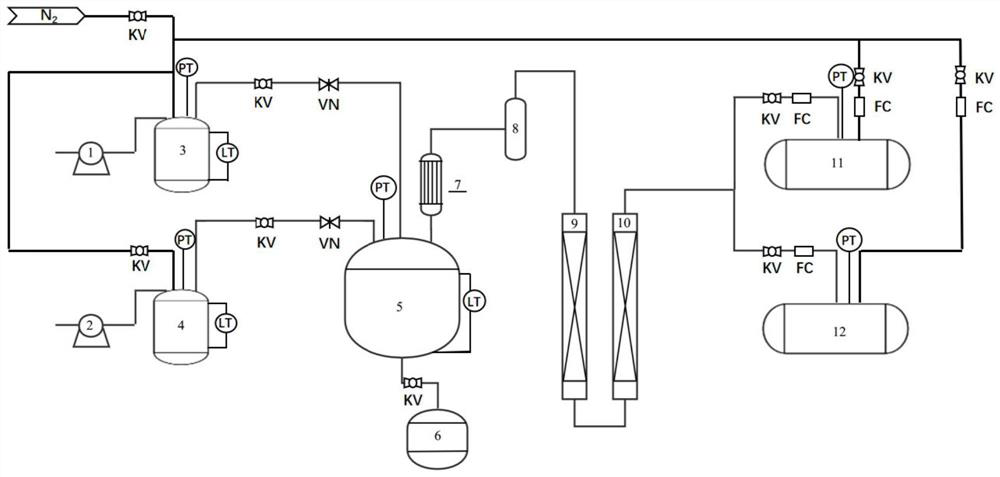
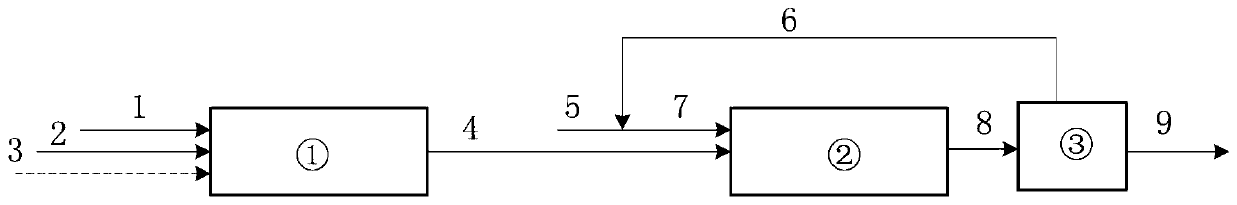
Method and device for fully automatically and continuously producing methyl nitrite
ActiveCN114805077APrevent liquefactionFully automatedProcess control/regulationDispersed particle separationAutomatic controlControl cell
The invention discloses a method and a device for fully automatically and continuously producing methyl nitrite. According to the method for continuously producing methyl nitrite, the liquid level, the temperature and the pressure in a reaction kettle are detected, and the detected liquid level value, the detected temperature value and the detected pressure value are remotely transmitted to a control unit; the control unit sends execution commands to valves in a pipeline between the raw material tank or the acid storage tank and the reaction kettle and a pipeline between the reaction kettle and the waste liquid tank according to preset conditions, and continuous reaction in the reaction kettle is realized by controlling the flow of a mixed solution of sodium nitrite and methanol and the flow of an acid solution. According to the method, through an automatic control system which comprises a detection unit, a control unit and an execution unit, the conventional intermittent operation concept is broken through, and automatic and continuous production of the methyl nitrite gas is achieved.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
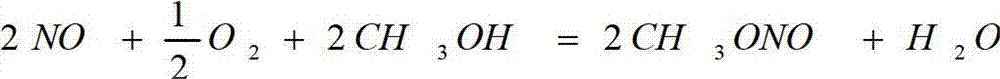
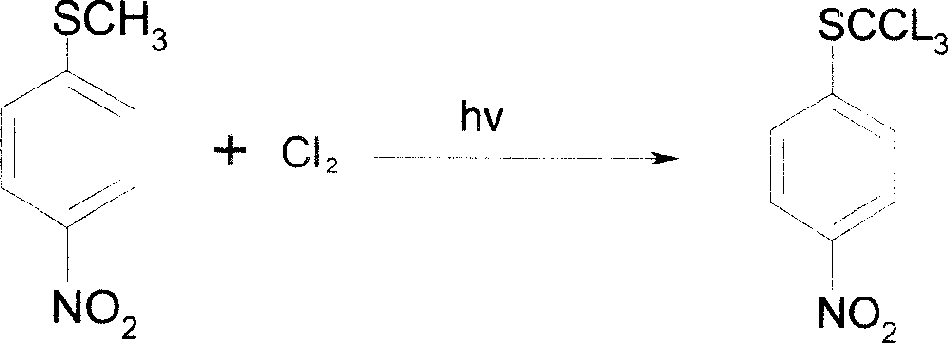
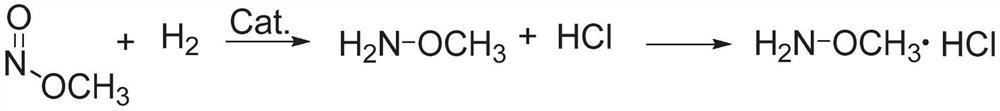

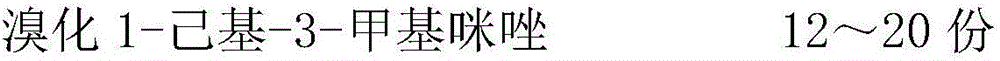

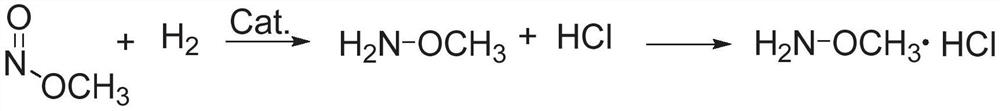
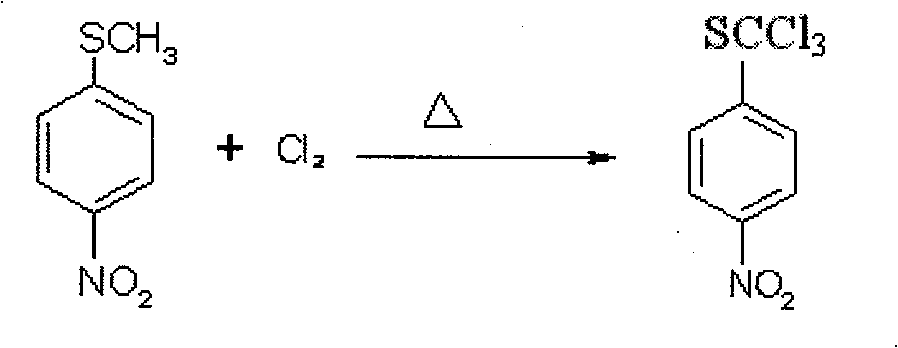


![Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)] methyl nitrate and processes of preparation thereof Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)] methyl nitrate and processes of preparation thereof](https://images-eureka.patsnap.com/patent_img/a9d1daa8-820e-4d38-9bc2-b095be16ac6d/US20160137686A1-20160519-D00000.PNG)
![Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)] methyl nitrate and processes of preparation thereof Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)] methyl nitrate and processes of preparation thereof](https://images-eureka.patsnap.com/patent_img/a9d1daa8-820e-4d38-9bc2-b095be16ac6d/US20160137686A1-20160519-D00001.PNG)
![Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)] methyl nitrate and processes of preparation thereof Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)] methyl nitrate and processes of preparation thereof](https://images-eureka.patsnap.com/patent_img/a9d1daa8-820e-4d38-9bc2-b095be16ac6d/US20160137686A1-20160519-D00002.PNG)