Three-degree-of-functionality terminal alkenyl energetic adhesive and synthesis method thereof
A technology with three functionalities and synthetic methods, which is applied in the direction of adhesive types, polyether adhesives, adhesives, etc., and can solve the problems of elastomers without energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
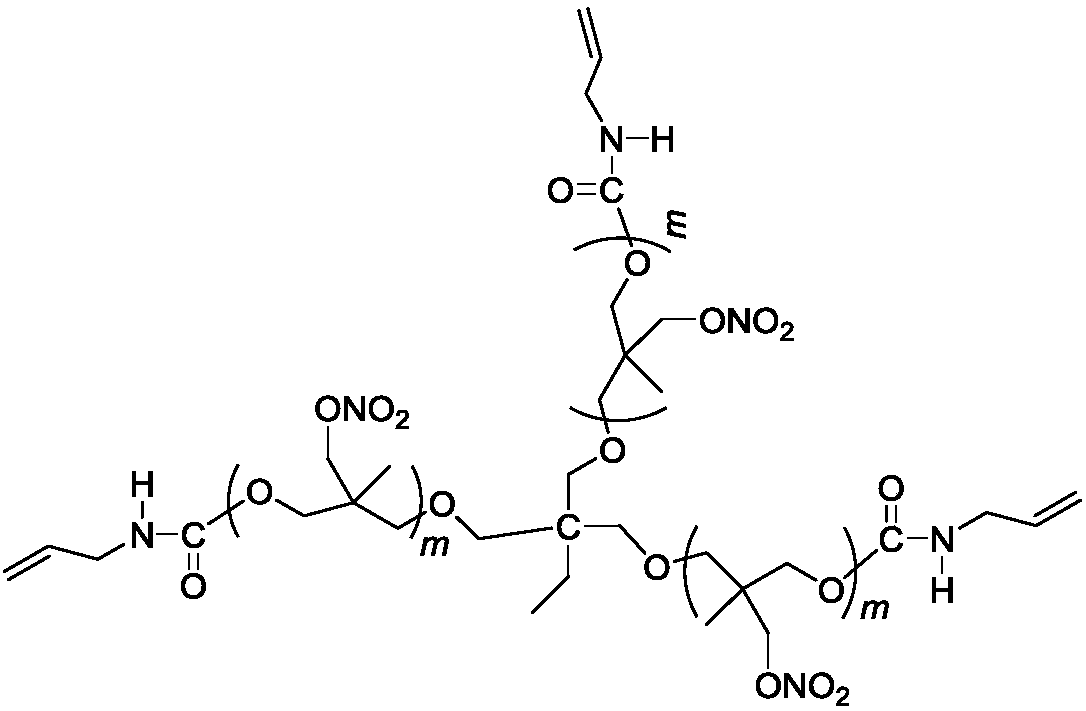
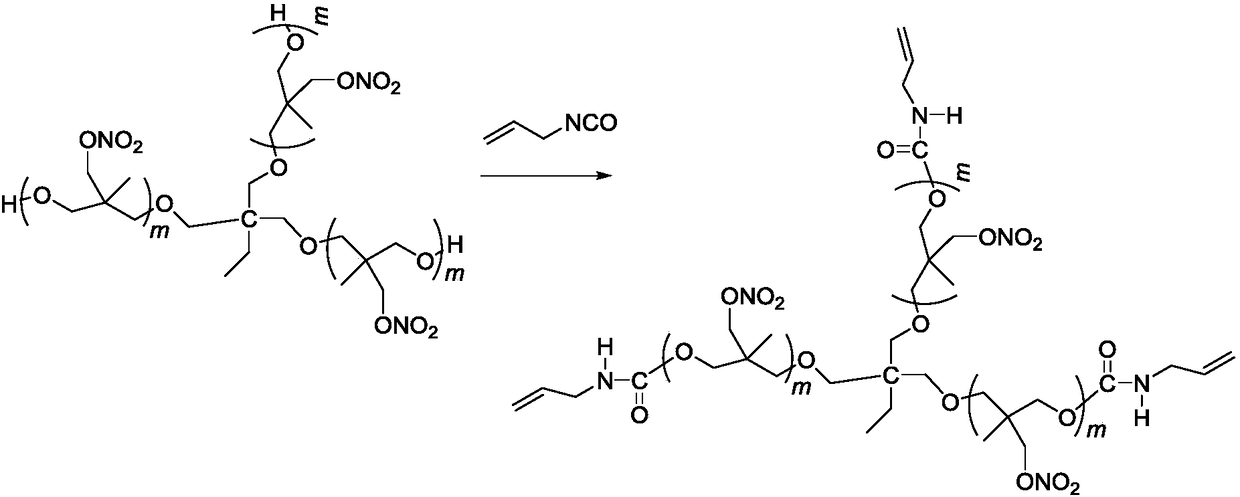
[0023] Add 22g (10mmol) trifunctional poly 3-nitrate methyl-3-methyloxetane in a four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer, dropping funnel, at temperature At 50°C, add 2.49g (30mmol) 3-propene isocyanate dropwise for 10 minutes. After the dropwise addition, raise the temperature to 77°C to continue the reaction for 12 hours, and remove unreacted 3-propene isocyanate under reduced pressure. 24.4 g of a pale yellow viscous liquid were obtained.
[0024] Structure Identification:
[0025] IR, ν max (cm -1 ): 3443 (-NH), 1727 (-C=O), 1518 (amide II peak C-N-H), 1112 (fatty ether C-O-C), 1632, 1281, 869 (-ONO 2 ).
[0026] 1 H NMR (CDCl 3 ,500MHz): δ5.70~5.80(m,1H),5.20~5.30(m,2H),4.31~4.49(m,12H),4.39~4.41(m,2H),3.25~3.37(m,24H) ,0.96~1.00(m,18H);
[0027] 13 C NMR (CDCl 3 ,125MHz): δ155.97, 134.37, 116.31, 75.84, 74.93, 73.77, 71.51, 66.55, 43.51, 40.40, 23.32, 17.33, 16.93, 7.51.
[0028] The number average molecu...
Embodiment 2
[0031] Add 22g (10mmol) trifunctional poly 3-nitrate methyl-3-methyloxetane in a four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer, dropping funnel, at temperature At 50°C, add 2.66g (32mmol) 3-propene isocyanate dropwise for 10 minutes. After the dropwise addition, raise the temperature to 77°C to continue the reaction for 18 hours, and remove unreacted 3-propene isocyanate under reduced pressure. 24.6 g of a pale yellow viscous liquid were obtained.
Embodiment 3
[0033] Add 22g (10mmol) trifunctional poly 3-nitrate methyl-3-methyloxetane in a four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer, dropping funnel, at temperature At 50°C, add 2.82g (34mmol) 3-propene isocyanate dropwise for 10 minutes. After the dropwise addition, raise the temperature to 77°C to continue the reaction for 18 hours, and remove unreacted 3-propene isocyanate under reduced pressure. 24.8 g of a pale yellow viscous liquid were obtained.
[0034] The application properties of the trifunctional alkenyl-terminated energetic adhesive of the present invention:
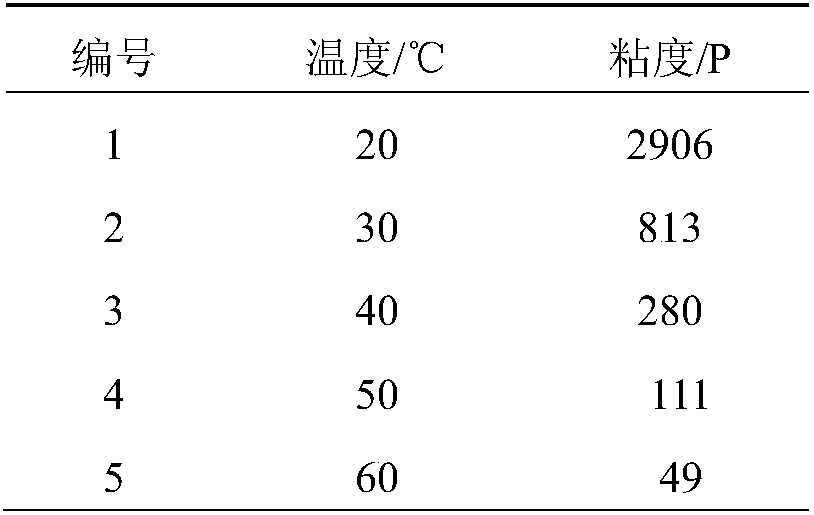
[0035] 1) Viscosities of trifunctional alkenyl-terminated energetic adhesives at different temperatures
[0036] The viscosities at different temperatures of the trifunctional alkenyl-terminated energetic adhesives prepared by the present invention are tested respectively, and the obtained data are as follows:
[0037] Table 1 Viscosities of trifunctional alkeny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com